Reply To:
Name - Reply Comment
Spotlight on Sputnik V

Residents in Kolonnawa receiving the Sputnik V vaccine
The general public started receiving AstraZeneca largely from mid February. They will be completing more than 16 weeks after their first dose by mid June
Since the frustration of these people is growing the Government will have to solve this problem and the best option would be to get 600,000 doses of AstraZeneca from a reliable source
The WHO in a statement said that it is not necessary to restart the full course of AstraZeneca vaccines if the second dose is delayed
But vaccine inequality has allegedly existed ever since Sri Lanka started receiving vaccines and speculation is rife that those in power forced their way to get both jabs of vaccines
In the event of a delay in the second dose of the Sputnik, it would be rational to have even partial immunity than no immunity
Frustration is growing among as many as 600,000 Sri Lankans awaiting the second dose of their AstraZeneca vaccine. With emerging COVID variants, medical experts warn that second doses of vaccines need to be given as soon as initial clinical protection starts waning off in order to boost immunity. While global vaccine production has already been challenged countries have been advised to follow a scientific process in administering vaccines. But vaccine inequality has allegedly existed ever since Sri Lanka started receiving vaccines and speculation is rife that those in power forced their way to get both jabs of vaccines, not only for them but for their kith and kin as well. From issuing chits based on political preferences to requests made by certain trade unions to get their immediate family members vaccinated, vaccine roll-out in Sri Lanka has taken an ugly turn. Recently, another incident of getting consent for one dose of Sputnik V vaccines was reported from Kandy.
vaccine. With emerging COVID variants, medical experts warn that second doses of vaccines need to be given as soon as initial clinical protection starts waning off in order to boost immunity. While global vaccine production has already been challenged countries have been advised to follow a scientific process in administering vaccines. But vaccine inequality has allegedly existed ever since Sri Lanka started receiving vaccines and speculation is rife that those in power forced their way to get both jabs of vaccines, not only for them but for their kith and kin as well. From issuing chits based on political preferences to requests made by certain trade unions to get their immediate family members vaccinated, vaccine roll-out in Sri Lanka has taken an ugly turn. Recently, another incident of getting consent for one dose of Sputnik V vaccines was reported from Kandy.
|
A seal on a consent form stating that the recipient agrees to get just a single dose of Sputnik V vaccines going viral on social media |
Expert committee approved decision
When inquired, Co-cabinet spokesperson and Media Minister Keheliya Rambukwella said that a special committee headed by virologists have approved to administer at least one dose of Sputnik V which is effective for around six months. “There were several recommendations on the administration of 50,000 doses of Sputnik V vaccine. The committee included Dr. S. M Arnold, Deputy Director General of Health Services, Dr. Nalika Gunawardena, National Professional Officer, WHO, South-East Asian region, Dr. Hasitha Tissera, Senior Medical Epidemiologist and Prof. Neelika Malavige, Professor of Immunology and Molecular Medicine, Faculty of Medicine at Jayawardenapura University,” said minister Rambukwella.
Emerging variants cannot be protected with first doses
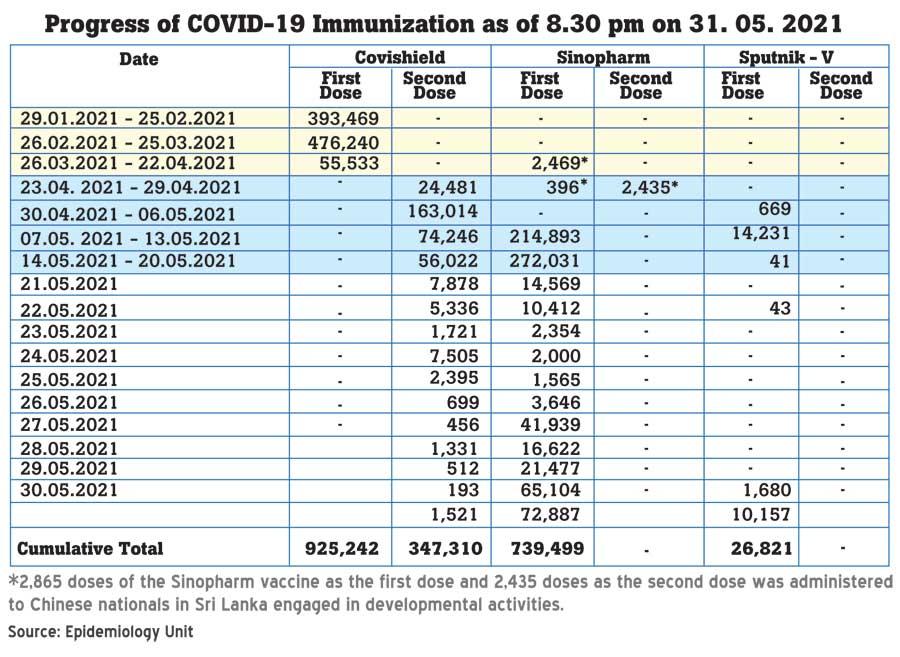
Shedding light on the number of vaccines Sri Lanka received so far, Association of Medical Specialists President Dr. Lakkumar Fernando said that initially Sri Lanka had received 500,000 doses of AstraZeneca vaccine as a donation and paid for another 1.5 million doses. “From that we got 500,000 doses. Another one million doses that we paid for are pending. Then we got 264,000 doses from the COVAX facility. Then we received 600,000 doses of Sinopharm and another 500,000 as a donation. We bought 15,000 doses of Sputnik V and received another 50,000 doses but these are only the first doses. Even though we expected to give second doses for those 15,000 after a gap of three to four weeks, those second doses never arrived.” said Dr. Fernando.
The Government then decided to buy another 14 million doses of the Sinopharm vaccine. “This is a good move because now there’s an assurance that around seven million people can be vaccinated with these doses. We have to give a second dose quite fast because unlike some other vaccines, Sinopharm doesn’t produce significant immunity with the first dose. Therefore, ideally the second dose should be given six weeks after the first dose.
“Out of just above 1.2 million AstraZeneca doses Sri Lanka received, a little above 900,000 people received the first dose, but only about 350,000 people could receive the second dose. As a result 600,000 are waiting to receive the Second dose. AstraZeneca was initially given to frontliners. The general public started receiving it largely from mid February. They will be completing more than 16 weeks after their first dose by mid June. Though there can be some protection against the original COVID variant after the first vaccine even up to 6 months protection against the rapidly spreading British variant and potential threat from the Indian variant maybe significantly inadequate after mid June for them; posing a real threat. Since the frustration of these people is growing the Government will have to solve this problem and the best option would be to get 600,000 doses of AstraZeneca from a reliable source. However according to confirmed reports the vaccines will arrive within the next 10 days. If we are to get it for plan B at a desperate situation by mid June we have to start getting ready now.” said Dr. Fernando.
There were several recommendations on the administration of 50,000 doses of Sputnik V vaccine”
- Media Minister Keheliya Rambukwella
Dr. Fernando said that one of the best scientific options for this is to consider using first dose of Sputnik V in place of the second dose of AstraZeneca. “The MOH should appoint a group of experts to study and discuss this possibility without delay and the best would be to do a clinical trial and also collect any scientific data available on such a mix and match on research done elsewhere. If this becomes the next available option we have to have enough first doses of Sputnik V available in the country to vaccinate the 600,000 people.
“But unlike Sinopharm, the delivery of Sputnik hasn’t happened as expected in the past,” he added. “The National Medicines Regulatory Authority approved Sputnik V on March 4, but even 15,000 vaccines arrived in the country more than one and a half months later; that is also without the second dose. The Government and the State Pharmaceutical Corporation will have to seriously look at the delivery schedule of Sputnik V if this solution is to be practical. Holding on to the Sputnik doses we get without using it as first dose for others for a very short period until these uncertainties are solved is important otherwise even if we try to make this mix and match option we may get stranded again without any Sputnik left to execute plan B. If that happens the next option would be to vaccinate these 600,000 with not one, but two doses of a completely different vaccine like Sinopharm. To do that we will have to wait for additional 600,000 doses. At the time there’s a shortage of vaccines.” he added.
However when people in Kandy arrived at the vaccination centre to get their first dose of Sputnik V they encountered a situation that raised questions. The consent forms which were in English, Sinhala and Tamil also had a seal with wordings “I agree to get only the first dose of Sputnik V vaccine.”
When asked about this incident Dr. Fernando said that it’s a very wrong and shortsighted decision.
“The Gamaleya Institute, manufacturer of Sputnik, claimed that the vaccine known as Sputnik Light which is identical to the first dose of Sputnik V, can be used as a single dose vaccine. However to date they have not provided scientific data to prove this claim. Though the two dose Sputnik V is approved in almost 40 countries, the WHO and stringent regulator approval is pending. However we do not know of any country or regulator that has approved Sputnik Light as a single dose vaccine yet though it is scientifically possible like in the Johnson and Johnson vaccine. Without the NMRA approval or approval of the advisory body on communicable diseases, the MOH deciding to use Sputnik V as a single dose undermines the regulatory requirement and creates a bad precedent. The best option the Government would have used in Kandy is to give people the Sinopharm under the prevailing circumstances,” said Dr. Fernando.
Though the two dose Sputnik V is approved in almost 40 countries, the WHO and stringent regulator approval is pending. However we do not know of any country or regulator that has approved Sputnik Light as a single dose vaccine yet” - Association of Medical Specialists President Dr. Lakkumar Fernando
However the Gamaleya Institute has reiterated that the first dose of Sputnik V can be used as a single dose vaccine following new clinical data. “This is a vague statement. Hearsay is one, submitting scientific data is another one. Even for the Sinopharm or any vaccine produced by a country, the regulatory process includes approval of properly submitted scientific data by an independent panel and approval by the official regulator and not the Director General deciding based on what a country has said without data,” he said.
The WHO in a statement said that it is not necessary to restart the full course of AstraZeneca vaccines if the second dose is delayed. Commenting on this statement, Dr. Fernando further said that the protection of AstraZeneca lasting for six months or beyond 12 weeks is known for the original or common coronavirus. “But there’s no scientific evidence to prove that this is the case for ‘variants of concern’ such as the British and Indian variants. The WHO is very cautious in making such statements, which is why for many months they said that the virus is not airborne at the onset of the pandemic and that wearing of masks by people without symptoms wasn’t recommended.” the doctor said.
Further reiterating on the use of the first doses of Sputnik V vaccines he said that if there’s any rationale to use Sputnik without keeping it, that should be for temporary outbreak control. “This could be at hotspots of sudden outbreaks such as in factories where there are young people who could develop immunity faster. But if that’s to be done, the way to do it is to explain the need with more transparency rather than being dodgy and unprofessional – a method or style frequently adopted by MOH. I personally feel that the political authority is not given all the necessary informational transparently by the relevant parties at MOH.” Dr. Fernando said.
Unethical to get consent for a single dose of a two-dose vaccine
“None of the Russian vaccines have been approved by the World Health Organisation,” opined Dr. Tush Wickramanayaka, Family Physician licensed in both Sri Lanka and UK. “When the President went and accepted the Sinopharm vaccines they weren’t approved by WHO. Then he said it will be used on Chinese nationals living here. Manufacturers can release any type of data, but it is the responsibility of any country to investigate it thoroughly. All vaccines have been approved for emergency purposes,” said Dr.Wickramanayaka.
“The manufacturing process of a vaccine has to pass four stages. At stage three they do clinical trials where the vaccine is tested on a population of over 1000 people. AstraZeneca and Pfizer vaccines were approved swiftly because the manufacturing process took place with WHO expertise. But the Russian and Chinese vaccines are manufactured on their own under their own research hence the WHO is taking time to analyze results. Sputnik V is a two-dose vaccine and nobody can say that one dose is enough,” she explained.
AstraZeneca and Pfizer vaccines were approved swiftly because the manufacturing process took place with WHO expertise. But the Russian and Chinese vaccines are manufactured on their own under their own research hence the WHO is taking time to analyze results. Sputnik V is a two-dose vaccine and nobody can say that one dose is enough” - Dr. Tush Wickramanayaka, Family Physician
“Research is done in controlled environments to determine efficacy of the vaccines. Here they would check the antibody levels after giving the first dose and at what point they wane off in order to give the second dose.
Efficacy shows how effective a vaccine is in a controlled state. Therefore it may show 100% efficacy. But in real life this case can change. Vaccines are transported from overseas and there maybe problems in transportation, storage, temperature conditions etc.
People receiving the vaccine may have varying nutrition levels and therefore its effectiveness will be between 95-98%. Therefore there’s a slim chance of contacting the virus and this is why you’re advised to maintain social distancing and wear a mask even after being vaccinated.” said Dr.Wickramanayaka.
The scientific brief by WHO regarding the delay in second dose of AstraZeneca vaccines starts off by saying that there’s insufficient evidence on clinical protection with the first dose beyond 12 weeks. “There’s no evidence to say if a second dose could be given after 6 months. Therefore, if we are going to give Sputnik V people should be given both doses and it’s unethical to get signatures from people for one dose only,” the doctor affirmed.
“Better to have partial immunity than no immunity under current circumstances” - Prof. Malavige
When asked if Sputnik V could be used as a single dose vaccine, Prof. Neelika Malavige, Professor in Immunology and Molecular Medicine, Faculty of Medical Sciences at Sri Jayawardenapura University said that currently there are phase 3 trials being carried out in Russia to find out the efficacy of the first dose of Sputnik V, which is an adenovirus 26 vector vaccine, in preventing COVID-19. “Since the trial is still in progress, there is no published data available regarding the efficacy of the first dose of Sputnik V used by itself in preventing COVID-19.” Prof. Malavige said.
Responding to a query on efficacy and effectiveness of Sputnik V vaccines Prof. Malavige said that there is no data available on the efficacy of the first dose of Sputnik V alone. “Many vaccines comprise two dose vaccines such as the MMR, chickenpox vaccine and many COVID-19 vaccines. The efficacy trials for Sputnik V, have been done using the 2 doses. However, from other vaccines such as the MMR and chickenpox vaccine, we know that children are protected from developing severe disease from these viruses after a single dose, although complete immunity only comes after administration of both doses.
For COVID-19 vaccines such as Astra Zeneca and Pfizer (both 2 dose vaccines), there is data showing that there is a certain degree of protection after a single dose, until the second dose is administered. Therefore, the first dose of Sputnik V, is likely to give some protection” - Prof. Neelika Malavige, Faculty of Medical Sciences at Sri Jayawardenapura
“For COVID-19 vaccines such as Astra Zeneca and Pfizer (both 2 dose vaccines), there is data showing that there is a certain degree of protection after a single dose, until the second dose is administered. Therefore, the first dose of Sputnik V, is likely to give some protection, although complete protection, that is shown in clinical trials (91%), is only likely to be achieved after administration of both doses.” the professor said.
We all know that we are in a pandemic situation, and there aren’t enough doses of vaccines to go around. Therefore, in the event of a delay in the second dose of the Sputnik, it would be rational to have even partial immunity than no immunity. I believe the current data from Sri Lanka shows, that only very few individuals who have even received a single dose of the Astrazeneca/Covishield vaccine have developed severe disease and died. Therefore, although based on current data, two doses are required for protection with the Sputnik V vaccine, given the extraordinary circumstances that we are in, it is better to have some immunity than no immunity,” she said.