Reply To:
Name - Reply Comment
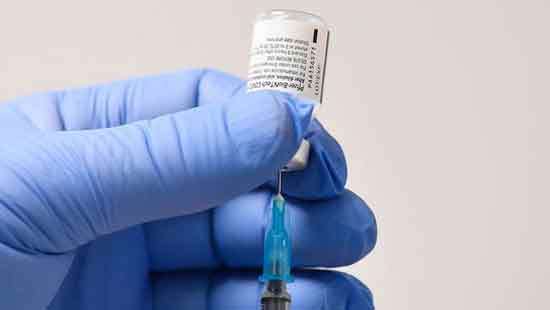
The Food and Drug Administration authorized Pfizer’s Covid-19 vaccine for emergency use on Friday, clearing the way for millions of highly vulnerable people to begin receiving the vaccine within days.
The authorization is a historic turning point in a pandemic that has taken more than 290,000 lives in the United States. With the decision, the United States becomes the sixth country — in addition to Britain, Bahrain, Canada, Saudi Arabia and Mexico — to clear the vaccine. Other authorizations, including by the European Union, are expected within weeks.
The F.D.A.’s decision followed an extraordinary sequence of events on Friday morning when the White House chief of staff, Mark Meadows, told the F.D.A. commissioner, Dr. Stephen Hahn, to consider looking for his next job if he didn’t get the emergency approval done on Friday, according to a senior administration official who spoke on condition of anonymity because he was not authorized to discuss the matter. Dr. Hahn then ordered vaccine regulators at the agency to do it by the end of the day.
The authorization set off a complicated coordination effort from Pfizer, private shipping companies, state and local health officials, the military, hospitals and pharmacy chains to get the first week’s batch of about three million doses to health care workers and nursing home residents as quickly as possible, all while keeping the vaccine at ultracold temperatures.(The New York Times)