Reply To:
Name - Reply Comment

Conveniences at our disposal also entail dangers and limitations—which we need to be aware of and take adequate precautions against. Decades ago we used firewood for cooking and oil for lighting lamps. Today firewood is partly replaced by Liquid of Petroleum Gas (LPG), and lighting by electricity.
adequate precautions against. Decades ago we used firewood for cooking and oil for lighting lamps. Today firewood is partly replaced by Liquid of Petroleum Gas (LPG), and lighting by electricity.
As people rush to enjoy modern facilities we often forget or pay little attention to their negative consequences. Apart from economic and environmental concerns, modern comforts create safety issues. Sometimes the business sector ignores safety concerns to sell their products and earn more profit. A culture of corruption encourages such practices.
These days there are growing reports suggestive of a spate of fire hazards and explosions originating from the use of LPG for cooking.
What is Liquid Petroleum Gas?
Crude oil refining yields a range of hydrocarbons (compounds comprising carbon and hydrogen) which are liquids or gases found under ambient conditions. Petrol, kerosene and diesel have boiling points (95-150); (150-350); (175- 375) degrees Celsius respectively—which are temperatures well above that of the natural environment. Additionally there are petroleum liquids that evaporate rapidly at room temperature. These are known as ‘ethers’.
Two important gaseous components derived from oil are butane and propane. When cooled, these turn into liquids at temperatures of -2 and -42 degrees Celsius. These gases can be liquefied at higher temperatures by applying pressure. At the average room temperature of 30 degrees Celsius, butane and propane can be liquefied at atmospheric pressures of approximately 2 and 10 atmospheres. These are approximately the minimum pressures needed to store butane and propane in liquid form at room temperature. But a larger percentage of propane or butane in a full cylinder exists as a liquid with the remaining 10 – 20% in the form of vapor. So when the cylinder valve opens, the gases (vapor) pushes out and the liquid inside slowly boils. Upon closing the valve the original pressure is regained as long as both liquid and gas are within and the value of pressure depending on the temperature and composition of the gas.
Meanwhile, LP gas also contains a small percentage of pentane (a hydrocarbon with a boiling point of 36 degrees Celsius). Like butane or propane, this substance too cannot cause any harm. Despite its higher boiling point, it is carried away with butane and propane at warmer temperatures.
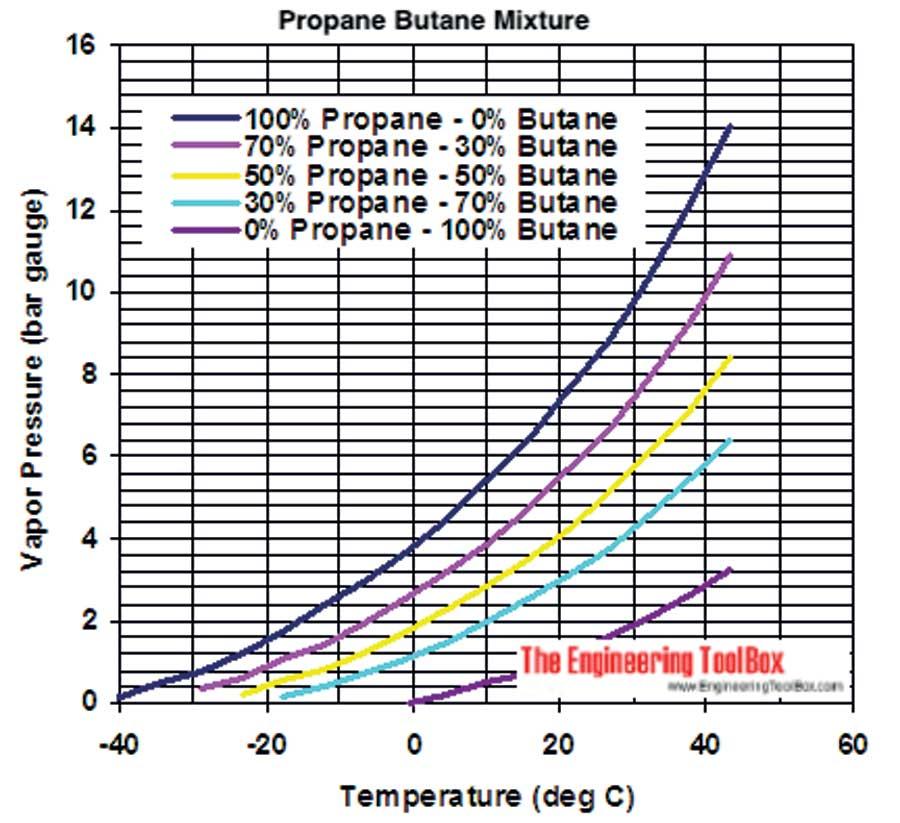
Commercial LPGs contain mixtures of butane and propane in varying proportions, compressed to pressures of the order of 7- 15 atmospheres. When the percentage of propane is larger, higher storage pressures are required. Again the vapor pressure inside the cylinder increases faster with temperature when the percentage of propane is increased. The plot (taken from www.engineeringtoolbox.com) presented below indicates how pressure varies with the propane/butane ratio in LPG (the numbers depicted in the vertical axis are approximately the pressures measured in units of normal atmospheric pressure).
"The cause of most accidents in the use of LPG for cooking has been gas leaks. A good precaution therefore would be to have a method of detecting leaking gas"
Generally LPGs used in warm tropical regions, contain 70-80% of butane and 30-20% of propane. Countries with freezing climates prefer LPGs containing a higher percentage of propane because butane evaporate too slowing under such conditions. In warmer environments LPG with a larger percentage of butane is preferable to prevent high pressure building up inside the containers.
It must be said that gas cylinders are sufficiently strong to withstand the pressures, irrespective of the amount of propane present. They don’t break-open and explode unless heated by a fire. However, if the pressure is high, gas could leak through a faulty valve in the cylinder or the burner. Sometimes connections to gas carrying tubing could also be leaky or completely yield when the pressure is excessive. LP gases are heavier than air, and if gas leaks in an enclosed space it builds above the floor. Such situations pose an imminent danger, because the slightest ignition trigger would lead to an explosion and fire.
When the percentage of LPG in the air exceeds 4-6%, the mixture could explode. Often this happens when someone attempts to light the burner. Sparks produced during the switching on or switching off of electricity could also ignite accumulated gas. Moreover, opening or closing metallic contacts inside a switch always create sparks. Friction encountered in working with tools and sparking due to static electrification are sufficient to ignite an LPG-air mixture. Propane is more flammable compared to butane.
Safety precautions
The cause of most accidents in the use of LPG for cooking has been gas leaks. A good precaution therefore would be to have a method of detecting leaking gas. The nose is the most sensitive chemical detector which humans and animals possess. Certain chemical substances in the minutest of quantities can be smelled. Propane and butane in pure form are odorless. Commercial LPG has a faint smell owing to the presence of aromatic impurities. But this is not strong enough to notice a gas leakage. Therefore an odorant is incorporated into LPG. Odorants introduced are sulphur compounds such as methyl sulfide, thiols and mercaptans. These compounds have smells resembling onions, leeks, durian or mella kola.
In many countries the addition of an odorant to LPG is a legal requirement that gas suppliers cannot ignore. It is ridiculous to say that a gas leak can be detected by the hissing sound of escaping gas. Such sounds are only heard when gas leaks out at an exceptionally high speed. Minute leaks in the cylinder valve and connections can be detected by smearing a solution of detergent. Blowing of bubbles is a sign of gas seepage.
Simple safeguards can greatly reduce accidents associated with LPG cooking. Always install the cylinder in an upright position in a well ventilated location to avoid a build-up of gas accumulation in the event of a leakage. After cooking disconnect the regulator from the cylinder. Do not leave burners unattended for long durations of time. Check the connections to tubing for tightness. If the cooker is not kept clean, ash and carbon could clog the burner valves causing malfunction. Also, salt in food corrodes burners and valves. If there is a sign of a gas leakage, do not ignite the burners and carefully disconnect the regulator from the cylinder. Open doors and windows, avoid switching-on or switching-off electricity. Stay away from the premises for a while. If there is a fire near the gas cylinder or tubes delivering gas to the burner, do not attempt to disconnect the regulator or shut the burner. Move far away and inform the Fire Department if the situation worsens.
Standard LP gas cylinders, cookers and accessories are designed to prevent gas leakages, even if there are pressure changes due to alterations in gas composition (propane/butane ratio) or changes in temperature. However, if the gadgets are substandard or faulty; gas leaks could occur, causing serious accidents.
To reduce the incidence of accidents associated with LPG cookers the general public must be vigilant and follow precautionary measures. The sale of brands of LPG without odorants and substandard cookers and accessories must be regulated. And any alterations to the gas composition must be notified. LPG can be managed safely by following the proper precautions and adopting consumer protection legislation.
The writer is a research professor and scientist attached to the National Institute of Fundamental Studies, Sri Lanka.