Reply To:
Name - Reply Comment

The healthcare sector has been facing a gamut of challenges including disparities and inequities in health outcomes, corruption, insufficient resources for health and a catastrophic and an impoverishing health expenditure among other issues
Incidents of medical negligence and cases of patients facing complications due to substandard drugs have flooded the news over the recent past. The government however is the main provider of healthcare to a vast majority of the population. This includes nearly 100% for preventive healthcare, 90% for in-patient curative care and 50% for curative out-patient care. This shows that the utilization of healthcare services for both in-patient and out-patient care is quite high. But as of late the healthcare sector has been facing a gamut of challenges including disparities and inequities in health outcomes, facilities and services, wastage, inefficiencies and corruption, insufficient resources for health and a catastrophic and an impoverishing health expenditure.
the news over the recent past. The government however is the main provider of healthcare to a vast majority of the population. This includes nearly 100% for preventive healthcare, 90% for in-patient curative care and 50% for curative out-patient care. This shows that the utilization of healthcare services for both in-patient and out-patient care is quite high. But as of late the healthcare sector has been facing a gamut of challenges including disparities and inequities in health outcomes, facilities and services, wastage, inefficiencies and corruption, insufficient resources for health and a catastrophic and an impoverishing health expenditure.
A worsening crisis
The COVID-19 pandemic and the economic crisis caused a disproportionate impact on the poor, vulnerable and marginalized communities. The shortage of medicine and the rising cost of medicines have resulted in high proportions of out-of-pocket expenditure which is currently over 50%. This is also known as catastrophic health expenditure. However, when several patients complained of weakened eyesight after getting a recommended eye drop and when a few others died under mysterious circumstances in hospitals, people started having doubts about the quality of medicines. As a result they delayed seeking treatments and this led to increased morbidity and mortality. Therefore people panic even when a routine side effect is being reported by the press.
On the other hand the lack of price controls and the near absence of monitoring private pharmacies result in arbitrary price fixing by pharmacy owners making it difficult for poor and vulnerable people to afford the cost of medicines. Therefore people refrain from taking the proper doses of medicines prescribed to them and skipping the frequency of medicines, once again leading to higher morbidity and mortality. One of the worst outcomes of people losing their trust on the health sector and the regulatory mechanism is that it provides a chance for more low quality drugs to flood the market.
There have been incidents of mass drug disasters reported from around the world but Sri Lanka didn’t have any such incidents as we were careful when registering medicines. If the manufacturer was small, or if we were unaware of them, we prevented them from sending drugs to our country
- Prof. Krishantha Weerasuriya Clinical Pharmacologist
Politicization of Sri Lanka’s healthcare system
However, there also exists a lack of knowledge among people when it comes to medicine. Medical experts opine that when people have good knowledge about a product, there’s less need for regulation. “But in the case of medicines, people have limited knowledge,” Prof. Krishantha Weerasuriya, Clinical Pharmacologist and former Advisor to World Health Organisation (WHO). “Therefore the government has to regulate medicines so that it’s available to the people. Safety, quality and efficacy are three essential elements when it comes to essential medicine. Since the 1970s Sri Lanka has universal health coverage and free medicine was part of that health coverage. Therefore the country could afford free medicine and healthcare,” said Prof. Weerasuriya.
Prof. Weerasuriya explained of instances when Sri Lanka imported food quality drugs and prevented substandard drugs from entering the country. “There was a problem drug from India at one point which caused liver problems. Rofecoxib is another drug which was registered in the UK but Sri Lanka waited as there were certain complications. The drug was later withdrawn. There have been incidents of mass drug disasters reported from around the world but Sri Lanka didn’t have any such incidents as we were careful when registering medicines. If the manufacturer was small, or if we were unaware of them, we prevented them from sending drugs to our country. This way we prevented major drug disasters.” said Prof. Weerasuriya.
As of late the National Medicines Regulatory Authority, the main body that regulates medicines came under heavy scrutiny for not delivering their tasks to ensure a quality healthcare system to the people. “From 1960 to 2020 there was a good system in place to check the quality of medicines although there were poor quality medicines that were procured from time to time. There was a technically competent staff that carried out the analyses and this was important in supporting universal health coverage. We were able to carry out these tasks back in the day and we could afford these facilities as well. This system began with Prof. Bibile and his legacy was taken forward by qualified administrators. But today we see administrators with no experience or knowledge in pharmacology taking decisions. Poor quality medicines are responsible for patient deaths. The Boards and Committees at NMRA have not been appointed,” said Prof. Weerasuriya.
Even though medicines regulation is a team effort, single-handed decisions would lead the way to corruption. “There are no checks on quality. Finally, tragically it’s the patients and the public who will pay final price of unavoidable suffering, disability and death,” he underscored.
The SPC needs new policy directions to do urgent purchases and that quality assurance needs to be determined through multiple mechanisms and governance. A high-powered committee should be appointed to decide on drugs required for the state health sector
- Dr. Ananda Wijewickrama Senior Consultant Physician
Non-essential priorities
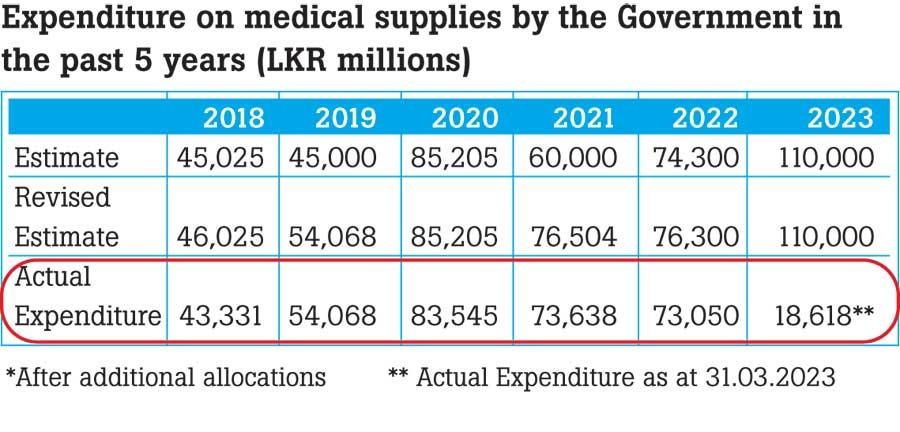
A survey conducted by the Pharmacology Association to analyze the reasons for the prevailing drug crisis reveals that the actual expenditure on medical supplies by the government was far less than the estimated expenditure during the last five years. According to the ABC Analysis, the number of non-essential items procured is far more than the vital and essential categories of medicines. “70% of the total cost has been spent on purchasing 31 essential medicines in category A, while 10% of the total cost has been spent on purchasing 318 non-essential medicines in category C,” said Priyadarshani Galappaththy, Professor of Pharmacology at the University of Colombo.
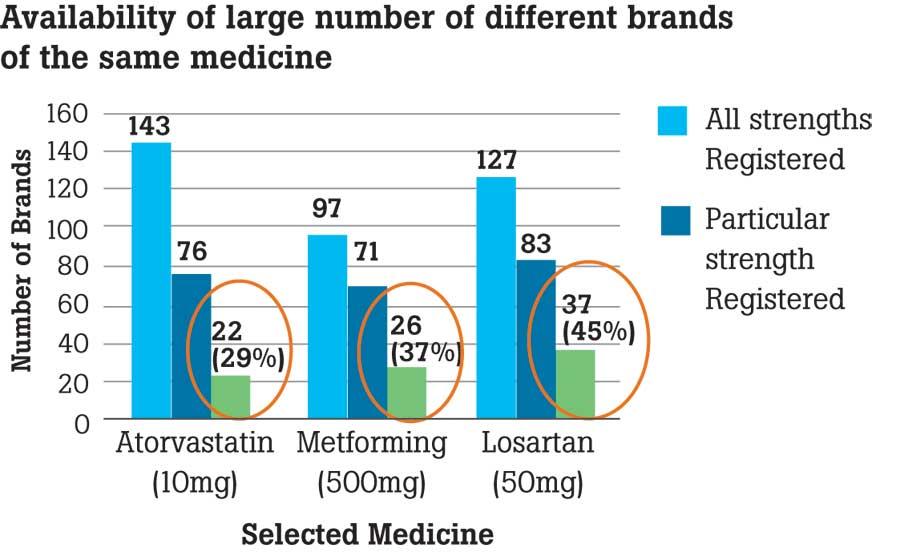
The matter of waivers of registration under the guise of emergency purchases had been subject to debate over the recent past. The analysis revealed that in 2022, 98 waivers have been granted while 130 of them have not been granted. She further said that a large number of different brands of the same medicine are also available in the market. For instance medicines such as Atorvastatin, Metformin and Losartan are some of the most sold drugs. “The NMRA includes the need and cost in addition to efficacy, quality and safety when it comes to registration,” said Prof. Galappaththy.
Speaking about quality testing in Sri Lanka, Prof. Galappaththy further said that the National Drug Quality Assurance Lab and State Pharmaceutical Corporation were testing about 1000 samples per year. But the National Medicines Quality Assurance Lab is carrying out a limited number of tests due to the shortage of staff and resources. Reportedly only 10 people are available to carry out tests and as a result post marketing tests too have reduced due to limited capacity.
Some of the key recommendations include establishing electronic systems to obtain all data on medicines including stocks, costs, purchases and so on; establishing a unit for monitoring the pharmaceutical situation with a Technical Advisory Committee from all specialist colleges; enhance the capacity for quality testing with increased staff, equipment and resources; minimizing waivers of registration and adhering to guidelines, avoid political interference in regulation and supply of pharmaceuticals and address concerns mentioned in Auditor General’s reports.
Other recommendations including addressing staff shortages at NMRA and NMQAL, evaluation of all submissions for registrations of essential medicines, improve coordination among NMRA, Medical Supplies Division and SPC during the procurement process, give priority to the purchase of essential medicines and monitoring high cost pharmaceuticals and local purchase items through hospital Drug and Therapeutic Committees.
The NMRA includes the need and cost in addition to efficacy, quality and safety when it comes to registration
- Priyadarshani Galappaththy, Professor of Pharmacology University of Colombo
Lack of transparency
The non-payment of money to pharmaceutical suppliers by the SPC, procuring medicines under the emergency clause, increasing prices of medicines, non-maintenance of medical equipment and migration of doctors and healthcare workers have aggravated the health crisis at present. Shedding more light on the process of registering medicines Dr. Ananda Wijewickrama, Senior Consultant Physician at the Infectious Diseases Hospital said that the evaluation of these medicines is being done by a Medicine Evaluation Committee (MEC). Drugs which are registered in reference countries such as the FDA in USA, MHRA in the UK are being expedited. But in the cases of waivers of registration it has been noted that most drugs are now being waived off sans approvals from the MEC.
Dr. Wijewickrama pointed out the case of issuing a waiver of registration for insulin. The price given for the tender was USD 1.56 per vial but the price given for emergency purchase through waiver of registration was USD 4 per vial. They even claimed that the emergency purchase is for a ‘buffer stock’.
He further showed how certain stocks of non-essential drugs have been purchased by referring to Swastha – an official website that comes under the purview of the Health Ministry. Dr. Wijewickrama further claimed that registered suppliers at the SPC have overdue payments and that they don’t apply for tenders. Those who apply for tenders are unregistered suppliers and once they get the tender, the waiver of registration is issued for importing the medicines.
In terms of solutions he said that all payments made to suppliers should be transparent as an initial step to regulating payments. “The SPC needs new policy directions to do urgent purchases and that quality assurance needs to be determined through multiple mechanisms and governance.
A high-powered committee should be appointed to decide on drugs required for the state health sector and the purchasing of recommended drugs should be done by the above committee. The backlog of payments for pharmaceutical suppliers should be done by the treasury in a uniform manner. Audits should be conducted on high cost medicines and medicines that are received via donations need to be assessed in a proper manner,” he said in conclusion.