Reply To:
Name - Reply Comment

- It’s noted that there were glaring lapses in the procurement process involving registration of companies with NMRA
- The National Audit has stated that the procedure followed by NMRA is against conditions specified in the Act
- The total difference between the highest bidder and the lowest bidder was approximately 400 million
- It was observed on certain instances that the delivery price of some drugs was more than 10 folds higher than the CIF price when examined
- A speedy system must be developed at NMRA to provide the speedy clearance of drugs
The country’s health sector has recently witnessed allegations of massive corruption involved in the purchase of medicinal drugs and medical devices as of now.
medicinal drugs and medical devices as of now.
High profile members of the health sector are accused of being involved in corrupt activities when purchasing medicine; done in the guise of obtaining emergency purchases.
As per Section 109 of National Medicines Regulatory Authority (NMRA) Act No: 5 of 2015, any medicine or medical device shall not be manufactured or imported without registering with the Authority and obtaining a license from the Authority. However, the Authority was vested with powers to issue letters of exemption from registration only for special cases such as to save a life, to prevent the spread of infectious diseases or epidemics, at national interest and national security.
Although the stipulated conditions of the Act is as such, the 2021 audit report issued by the National Audit Office on NMRA, reveals how letters of exemption for registration had been issued to the State Pharmaceutical Corporation (SPC) and private institutions to import 67 medicines and 140 medical equipment with claims made that it was done due to cancellation of registration, lack of registered suppliers, etc., which do not fall under such circumstances.
In response to the Audit findings the NMRA says that it doesn’t agree with the observation made by the National Audit. According to NMRA, actions have been taken in terms of Section 109 of the Act, with the approval of the Ministry of Health, in special cases such as to save a life or to prevent the spread of an epidemic disease, cancellation of registration, non-availability of registered suppliers, non-importation of medicines and occurring crisis situations due to shortage of medicines in public and private sector due to the declining trend of registration.
Audit. According to NMRA, actions have been taken in terms of Section 109 of the Act, with the approval of the Ministry of Health, in special cases such as to save a life or to prevent the spread of an epidemic disease, cancellation of registration, non-availability of registered suppliers, non-importation of medicines and occurring crisis situations due to shortage of medicines in public and private sector due to the declining trend of registration.
However the National Audit has stated that the procedure followed by the NMRA is against the conditions specified in the Act.
Although as there is no epidemic nor emergency situation in the country at present, questions have been raised as to why the Ministry of Health is continuing with emergency purchases. Officials of the Ministry of Health allege that these purchases are done to enjoy commissions.
According to the Ministry sources, who wished to remain anonymous, there are many instances that they can underscore how emergency purchases have been done with the knowledge of the Ministry of Health, State Pharmaceuticals Corporation (SPC) and the NMRA without following procurement procedure; even though there are ample stocks with the Medical Supplies Division (MSD).
“Whenever we inquire as to why available medicines are purchased under ‘emergency purchase’ category, we are told that if a tender is floated, it will take at least more than 10 months to get the necessary stocks to the country and that was why emergency purchases are done,” sources said.
Although the health ministry officials’ stance is such, this newspaper is in the possession of details of official documents to prove how the tender that was floated in 2022, to purchase Cefuroxime Sodium Injection - which is used as an antibiotic - was cancelled by the Cabinet of Ministers. The cabinet ministers were in favour of the highest bidder though the Finance Ministry directed to offer the tender to the lowest bidder.
The total difference between the highest bidder and the lowest bidder was approximately Rs.400 million, but by cancelling this tender, another emergency purchase had been done and the tender was awarded to two companies - one being the local agent of the earlier highest bidder and the total loss to the country by not awarding the contract to the lowest bidder in the cancelled tender is approximately Rs. 1, 200 million.
“When the country is facing a financial crisis, is it justifiable to procure a single product at a cost difference of over Rs 1, 200 million burdening the tax payers?” sources queried.
Finance Minister’s suggestions ignored
Despite the observation made by the Finance Minister on May 5, 2023, to award the contract for three months to the bidder who quoted the lowest, the Cabinet of Ministers as per the request made by the Health Minister through the cabinet memo dated May 8, 2023, did not accept the Finance Minister’s suggestion and decided to cancel this tender and call for fresh bids.
Finance Minister, President Ranil Wickremesinghe, made his observation based on the non-availability of stocks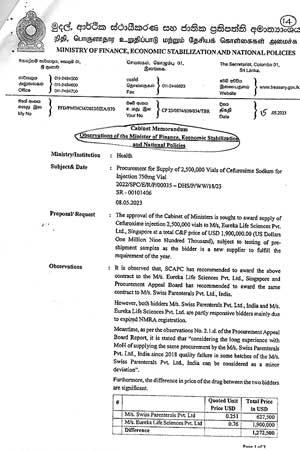 with the MSD and considering the price difference between the lowest and the highest bids. Following Finance Minister’s suggestion, the Health Minister in his cabinet memo has stated that his opinion on the supply of quality medicine is purely based on the NMRA reports which deal with the quality and registration of site and product. Hence the bidder that was recommended has no NMRA registration and cannot be considered as the responsive bidder.
with the MSD and considering the price difference between the lowest and the highest bids. Following Finance Minister’s suggestion, the Health Minister in his cabinet memo has stated that his opinion on the supply of quality medicine is purely based on the NMRA reports which deal with the quality and registration of site and product. Hence the bidder that was recommended has no NMRA registration and cannot be considered as the responsive bidder.
The bidder that was selected by the Department Procurement Committee (DPC)/Technical Evaluation Committee (TEC), the Procurement Appeal Board (PAB) and the Ministry Procurement Committee (MPC) has quoted US$ 0.251 per vial and the bidder that was recommended by the Standing Cabinet Appointed Procurement Committee (SCAPC) has quoted US$ 0.76 per vial. The price difference of the two bidders was US$ 0.509 per vial. The total price difference for 2.5 million vials is US$ 1,272, 500 which is approximately Rs. 600 million.
In October 2022, international competitive bids were called (tender No: 2022/SPC/E/R/P/00035 – DHS/P/WW/18/23) to supply 2.5 million vials of Cefuroxime Sodium which is an antibiotic injection to treat wide variety of bacterial infections.
Two bidders were selected as partly responsive bidders by the DPC/TEC. The TEC suggested to test pre-shipment samples of both bidders M/s Swiss Parenterals Ltd, India- the lowest bidder and M/s Eureka Life Sciences, Singapore - the highest bidder.
Swiss Parenterals Ltd had been supplying this medicine to SPC since 2018 and has the European GMP (EUGMP) accreditation for its manufacturing site. “EUGMP certification is globally accepted and is a higher level regulatory body than the local NMRA,” Health Ministry sources claim.
According to the sources, Swiss Parenterals Ltd was considered as not responsive by the SCAPC, as there were three instances where quality failure has been reported from three batches and they did not have the NMRA registration at the time of the open/close the bid. According to the sources, it is questionable to accept these quality failed reports as they have been issued by the NMQAL (NMRA laboratory) which is not an accredited lab by the Sri Lanka Accreditation Board.
“This company has provided over 5.5 million vials to the MSD since 2018 and the samples have been tested by accredited laboratories which are accepted by the SPC. All these laboratories have approved the quality of this medicine. It is a common industry knowledge that a pharmaceutical product fails not only due to manufacturing defects, but due to poor handling, storage, transport and possible sabotage as well,” sources said.
accredited laboratories which are accepted by the SPC. All these laboratories have approved the quality of this medicine. It is a common industry knowledge that a pharmaceutical product fails not only due to manufacturing defects, but due to poor handling, storage, transport and possible sabotage as well,” sources said.
Sources also said that neither the NMRA nor NMQAL has ever confirmed that these quality failures are explicitly due to the manufacturer’s fault.
Although Minister Rambukwella in his cabinet memo states that his opinion on the supply of quality medicine is purely based on the NMRA documents and reports which deal with the quality and registration of site and product, the health ministry sources questioned as to why the SPC then awarded Swiss Parenterals, India, a tender (No: SPC/AM/109/ 2022) in July 2022, to supply 90, 000 vials of the same medicine although they did not have the NMRA registration in place.
“Why did the Ministry of Health award a tender in July last year to the same company to supply 90,000 vials even though they did not have the NMRA registration? At this procurement why did the Health Ministry follow a different stance? We believe all this is due to the commission promised to the officials,” sources alleged.
After considering the details provided regarding the tender, the MPC on January 27, 2023, decided to award the procurement to the lowest bidder Swiss Parenterals, in view of the significant price difference between the two bidders, subject to obtaining Waive off Registration (WOR) and to obtain independent lab reports for pre-shipment samples. However, Secretary Ministry of Health did not endorse the MPC decision stating that the lowest bidder isn’t responsive.
This report was then referred to the Standing Cabinet Appointed Procurement Committee (SCAPC) where the Health Secretary was a member. At the SCAPC meeting held on February 22, 2023, it was decided to award 50% of the total quantity to the highest bidder Eureka Life Sciences, Singapore at a rate of US$ 0.76 per vial.
However, Committee Member of the SCAPC, Additional Director General, Department of Treasury Operations, Ministry of Finance, Mr. R.M.D.K.G.N.B. Ranatunga didn’t agree with the decision taken.
He states that he does not agree with the SCAPC decision as per the TEC report; which was decided upon to obtain WOR and an independent lab report before pre-shipment samples, which has not been considered.
According to the ministry sources an appeal was lodged with the Procurement Appeal Board on March 24, 2023 by the local agent of Swiss Parenteral’s India, P.T.C. Medical (Pvt) Ltd.
The PAB which met on April 11th and 17th, 2023, has observed that the TEC has considered both Swiss Parenterals and Eureka Life Sciences as partly responsive bidders due to expiration of their NMRA registration certification and a decision was delivered on April 18, 2023, recommending to award the contract to Swiss Parenterals since it is the lowest evaluated substantially responsive bidder.
The report further states, ‘the appellant making representations against the determination of the award states they applied for the registration of the new manufacturing site in India in 2016. After an extensive delay, in 2020, NMRA granted a provisional manufacturing site registration as the said site was already certified with EUGMP. This provisional registration was granted for one year which expired in 2021.
‘Since several requests were made to NMRA for Good Manufacturing Practice (GMP) for a visit, this has been postponed owing to the Covid-19 pandemic and for other reasons. This led to delays in getting product registration of the Cefuroxime Injection. After a two-year delay, NMRA finally carried out a GMP audit on the manufacturing site on March 15, 2023, and declared that the site meets the expected standard.
MPC’s recommendations rejected
‘Having rejected the recommendations of the MPC, Secretary Ministry of Health – a member of the SCAPC has recommended awarding the contract to the next partly responsive bidder for higher price without valid reasons. It is to be noted that the SCAPC has not given any reason to reject the recommendations of the DPC/TEC and MPC. The PAB further observes that the SCAPC has arrived at the said decision without having the TEC of the SCAPC.
‘Since the lowest bidder and the SCAPC recommended bidder are partly responsive bidders mainly due to expired NMRA registration, PAB observes that both bidders are equal players.
‘Considering the long experience with MOH of supplying the same injection by the appellant’s company- Swiss Parenterals since 2018, quality failure in some batches can be considered as a minor deviation and could be rectified based on the recommendations of the MPC and DPC by obtaining WOR and independent lab reports for pre-shipment samples as stated above.
‘Considering the stock out situation with the MSD of the MOH, PAB recommends to award the supply of the said vials to Swiss Parenterals, since they are the lowest evaluated substantially responsive bidder for the said procurement’.
Following the SCAPC decision, a cabinet memo was submitted by the Health Minister Keheliya Rambukwella on May 8, 2023, seeking approval of the Cabinet of Ministers to award the contract to M/s Eureka Life Sciences, Singapore at a total price of US$ 1.9 million subject to testing of pre-shipment samples as the bidder is a new supplier to fulfill the requirement of the year.
However, Minister of Finance, Economic Stabilization and National Policies on May 15, 2023, submitted a cabinet memo (Ref: No: PFD/ PMD/ CM/ 2023/ HEA/170) suggesting to award the contract to Swiss Parenterals Pvt Ltd.
The Finance Minister’s observations further state, ‘It is observed that the SCAPC has recommended to award the above contract to M/s Eureka Life Sciences Pvt Ltd, Singapore, but the PAB has recommended to award the same contract to Swiss Parenterals Ltd, India.
‘However both bidders are partly responsive bidders mainly due to expired NMRA registration.
‘Meantime, as per the observations No.2.1.d of the PAB report, it is stated that considering the long experience with MoH of supplying the same procurement by Swiss Parenterals since 2018, quality failure in some of those batches can be considered as a minor deviation.
‘Further the price difference of the drug between the two bidders are significant and the total difference is US$ 1.2725 million. Accordingly, the cost difference between the two drug consignments is approximately Rs.400 million for the total quantity.
‘It is noted that glaring lapses in the procurement process involving in NMRA registration, monopoly and oligopoly situations with regard to certain drug imports, weak procurement planning etc, has led to dramatic increase in the cost of medical supplies to the MSD. It is observed that on certain instances the delivery price of some drugs was more than 10 folds higher than the CIF price when examined. This situation has arisen due to handling the medical supplies by a cartel of suppliers who have affiliation to each other. Due consideration has to be given by the NMRA when registering the suppliers in order to create sufficient competition. An appropriate systems should be developed at NMRA to provide speedy clearance for drugs. Fully integrated MIS should be developed between the MSD and the SPC to minimize delays in procurements.
‘However, considering the stock position of the said injections at the MSD, and the PAB recommendations, it is suggested to award the contract for three months requirements to Swiss Parenterals Ltd, India.
‘Further, the MoH should take speedy action to recall the fresh bids for the same injections by following the guidelines for Health Sector Emergency Procurement Process’.
Despite the Finance Minister’s directive, the Cabinet of Ministers at its meeting held on May 23, 2023, cancelled the procurement and directed the Secretary Ministry of Health to take action expeditiously to invite new bids for the supply of the same.
Meanwhile, Health Ministry sources said how new conditions were laid when calling for fresh bids to prevent M/s Swiss Parenterals India from sending their quotations for the second tender.
“This was the Ministry of Health’s ploy from the initiative. They did not want to award the tender to the lowest bidder as it is alleged they have been promised a huge commission from the highest bidder,” sources added.
This newspaper is in possession of details of the purchase order dated June 28, 2023 issued to Yaden International (Pvt) Ltd of 67-Norris Canal Road, Colombo 10- who is the local agent of M/s Eureka Life Sciences Pvt Ltd, Singapore at a rate of Rs.589 per vial.
“At a time the country is facing a huge financial crisis, the questionable tender has been offered to the former failed bidder’s local agent at a very high rate and the difference between the quoted price of Swiss Parenteral of India and Yaden International (Pvt) Ltd is Rs.511.20.
All attempts to contact Secretary Ministry of Health, S. Janaka Sri Chandraguptha failed as he did not respond to any of the calls. Although a text message was forwarded seeking a comment on the allegations levelled against the MOH, Chandraguptha did not send a comment until the paper went for publication.