16 Mar 2021 - {{hitsCtrl.values.hits}}

Health Minister Pavithra Wanniarachchi taking a spoonful of Dhammika Peniya
 Having spent our childhood with Asterix comics, we were not really surprised in encountering our own Getafix (‘Veda Pappa’ in Sinhalised version) in the form of a house constructor turned medic in Kegalle, offering us magic portion in quarter litre bottles, a proven remedy, according to him, against COVID-19, the worst known pandemic of recent times.
Having spent our childhood with Asterix comics, we were not really surprised in encountering our own Getafix (‘Veda Pappa’ in Sinhalised version) in the form of a house constructor turned medic in Kegalle, offering us magic portion in quarter litre bottles, a proven remedy, according to him, against COVID-19, the worst known pandemic of recent times.
A point worth noting: He is not a registered ayurvedic practitioner. Neither is he a part of any established medical system in the country. Still he created a wonder product that was later to be branded by his own name; Dhammika Peniya.
With villagers gathering for a sip, not too different from the manner Gauls queued up in 50 BC, our own ‘Veda Pappa’ treated everyone generously, be them his own neighbours or reputed bacteriologists and virologists. The last part is an exaggeration. No virologists were reported visiting him, but many qualified medics actually did. And they were also ready to vouch for the efficacy of the medicine, which now awaits proper clinical trials before scientifically approved by the medical community. Whatever inside those bottles surely has super powers, said local media, which is supposed to have double impact: As a COVID-19 drug and a vaccine. Once taken, you will not catch COVID-19 for life.
Drama almost over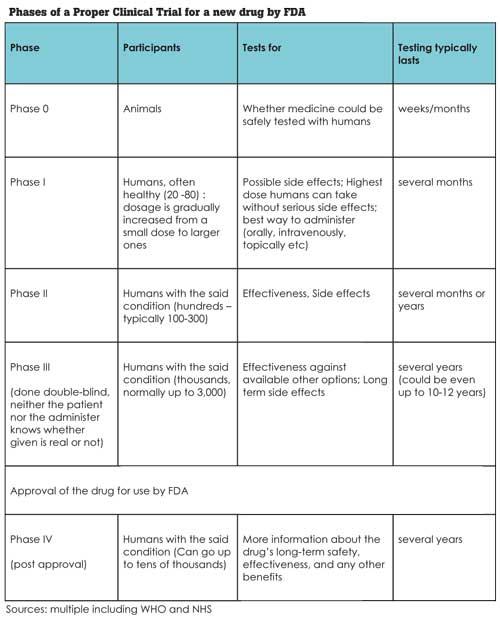
It looks like the drama is almost over now. With the island wide vaccination programme, whatever the limited interest masses had on D-Peniya has worn out. The most gullible will continue to use it - there still exist flat earth societies - but the glamour is gone. Still some questions remain unanswered. Was it a successful exercise? Can somebody replicate the business model in future? Or should we forget the entire episode treating it as a bad joke?
Manufacturing our own drugs is indeed great. I am sure there would be a great international market for a COVID-19 drug with at least 50 percent success rate. (Kegalle drug claimed 100 percent) Imagine the number of countries ready for an immediate purchase.
With the going price of Rs. 6,500 (about US$ 35), international orders for one billion bottles would yield US$ 35 billion – one third of Sri Lanka’s GDP and seven times Sri Lanka’s aggregate annual earning from tourist industry. Now we sense something wrong. Big dreams. Too big. But the obvious question: Will such a business model really work?
Appearance of ‘local super drugs for COVID-19’ and political approval for them look common for this part of the world. Few months back Indian PM Modi was at the receiving end when scientists questioned his backing of a ‘traditional medicine’ known as ‘kadha’ which will “increase immunity.” The medical experts claimed there is no way the body immunity can be improved in this manner. Still that has not prevented India’s Ministry of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH) promoting ‘traditional healing therapies’ against COVID-19. At least, that came from a somewhat established system.
Let’s leave it at that for the moment. Searches for COVID-19 drugs and treatments are not uncommon. NY Times follows the development of 22 of most-talked-about treatments for the COVID-19. Its analysis provides a valuable insight on COVID-19 treatment landscape from reasonably promising to almost useless drugs and treatments.
An example. Silver compounds treating COVID-19 is a myth. Lozenges, lotions and soaps containing silver, are not effective though some metals do possess natural antimicrobial properties. National Institutes of Health of USA warns that “scientific evidence doesn’t support the use of colloidal silver dietary supplements for any disease or condition.” It can also be dangerous, it states further, causing people’s skin to turn blue and making it difficult for them to absorb antibiotics and other drugs.
The analysis also focuses on some herbal remedies. What is known as Oleandrin is a compound produced by the oleander shrub. The plant extract is difficult to digest and can be dangerous to humans. With many plant compounds, even some potentially lethal ones, being medically useful, researchers have investigated Oleandrin as a potential treatment for COVID-19. The experiments were inconclusive. Other researchers have released a study finding that it was effective in a culture of monkey kidney cells infected with COVID-19. The study has not yet been published in a scientific journal. So the treatment hangs on. This is a good example that the world does not ignore herbal solutions. Still to be taken seriously they should first prove themselves.
Many drugs being researched
In fact, contrary to what was reported by the Sri Lankan media, that Sri Lanka is the only country to produce a remedy for COVID-19, more than 150 different drugs are being researched in clinical trials in different countries. Most of them are already existing drugs that are being trialled against the virus.
For example, UK is running world’s largest clinical trial, called Recovery, with more than 12,000 patients taking part - it is one of the few trials to have given a definitive view on which drugs do and do not work and WHO is running the Solidarity trial to assess promising treatments in countries around the world. Multiple pharmaceutical companies too are running trials of their own drugs. Still no magic bullets, such as our own D-Peniya, have been found.
Why? Good Question. A clinical trial is not a simple process. These research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention can take many years. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment. The process followed in USA before (and after) Food and Drug Administration (FDA) approval of a new drug or treatment has multiple phases and is substantially complicated. Still it is essential the procedure is followed to the letter. We talk about human lives here.
Then another important point. Diseases spread by virii, from the common cold to COVID-19, are so difficult to treat we almost believe that they have no cure. Of course, bacterial diseases are easy to treat. Bacteria are living elements, virii are not quite.
Antibiotics, which are used to fight bacterial infections, attack the bacteria’s cell walls, block protein production and stop bacteria from reproducing. Compared to bacteria, virii are minuscule. Bacteria live on their own, virii ‘live’ on host cells, having none of the hallmarks of living things such as a metabolism or the ability to reproduce on their own.
No eradication
More to this. Even when antiviral drugs are available for some virii, they do not necessarily cure the infection. For instance, there are HIV drugs effective at suppressing virus replication but they don’t eradicate it. Seasonal influenza can be treated with an antiviral medication, which can help shorten the duration of the illness, but it’s common to be able to detect the virus even after a patient recovers. Perhaps the only virus disease that can truly be cured by drugs is hepatitis C. The rest are extremely harder to target with drugs.
Drugs do help, though, in some circumstances. Remdesivir, also known in its brand name Veklury, has been the only drug to gain approval from the FDA for COVID-19 treatment. It works by interfering with the creation of new virii, inserting itself into new viral genes. A drug originally tested as an antiviral against Ebola and Hepatitis C, only to deliver lackluster results now appears to be more effective against COVID-19.
It can stop COVID-19 from multiplying in cells. Sufficient clinical trials were done. The drug was found to reduce the recovery time from 15 to 11 days and was first approved by FDA in May 2020 and then extended after another study found that patients with less severe forms of COVID-19 seemed to benefit modestly from a five-day treatment course of the same. Still this is an exception; not the norm.
Requires rigorous testing
This provides us ample reasons to suspect when somebody with no medical background proposes a rudimentary herbal drug for a complicated virus disease like COVID-19. Certainly that should be rigorously tested. The testing must satisfy two conditions: Firstly, the drug should be free from short and long term side effects. Then it should be effective. No point wasting time over any ‘remedy’ that does not meet these two conditions.
Going by what happens, I guess the most worried should be the genuine indigenous and ayurvedic drug manufacturers and vendors. It is the credibility of the medical system that is at stake. Normally indigenous and ayurvedic medicines are prepared by qualified professionals within their own systems. They are also tested clinically, though the process may not be as strict as it is supposed to be. Approval and usage of unlisted and completely new drugs with questionable effectiveness cannot be put into the same basket. Certainly not ones created by those with little or no prior medical background.
Were somebody’s sole objective to earn a quick buck, ethically or unethically, it could have been a good business case. D-Peniya did bring instant results. To get the same publicity any other product would have to spend millions in advertising. D-Peniya received it with virtually no cost. The customers too were satisfied. Absolutely no complaints. Even when they caught COVID-19 later the customers were happy to put the blame on themselves rather than on the manufacturer. They have not used the ‘medicine’ with correct instructions, they themselves admitted. If we see from this angle, yes, no doubt it was a good business case - something one would try replicating if one has the investment and few good political connections.
Only the business ethics make it a poor show. In business, we normally don’t claim a product what it isn’t, whether customers accept it or not. Were it branded a local medicine raising the immunity of the body, the claim could have been more acceptable - many herbal medicines do so to varying extents. That would have not been off the beaten track though. Its creator wanted a spike in marketing and a spike it was. Still no MBA textbook would offer it as a business case study worth studying, we guess.
(Chanuka Wattegama [email protected] is an academic and a policy researcher. Opinions articulated here are personal)
23 Dec 2024 8 hours ago
23 Dec 2024 23 Dec 2024
23 Dec 2024 23 Dec 2024
23 Dec 2024 23 Dec 2024