05 Mar 2021 - {{hitsCtrl.values.hits}}
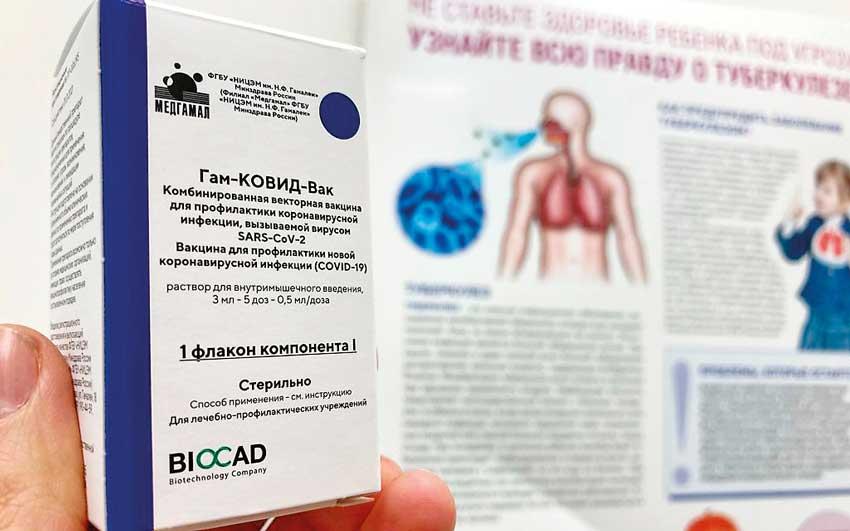
Amsterdam (dpa), 4 March, 2021-The European Medicines Agency (EMA) is launching a fast-track procedure to test the Russian coronavirus vaccine Sputnik V.
The decision to proceed was based on results of laboratory tests and clinical trials in adults, the EMA announced in Amsterdam on Thursday.
According to the studies, Sputnik V stimulates the formation of antibodies against the virus and can help protect against the respiratory disease it causes, Covid-19.
The EMA experts will evaluate the efficacy of the vaccine according to the rapid “rolling review” procedure. This means that test results are already being examined, even if not all results are available yet and no application for marketing authorization has been submitted. The Russian vaccine is already being used on the public in several countries outside Russia. Some EU countries have said they want to use it even if there is no official EU approval.
15 Nov 2024 16 minute ago
15 Nov 2024 1 hours ago
15 Nov 2024 2 hours ago
15 Nov 2024 2 hours ago
15 Nov 2024 2 hours ago