04 Jun 2021 - {{hitsCtrl.values.hits}}
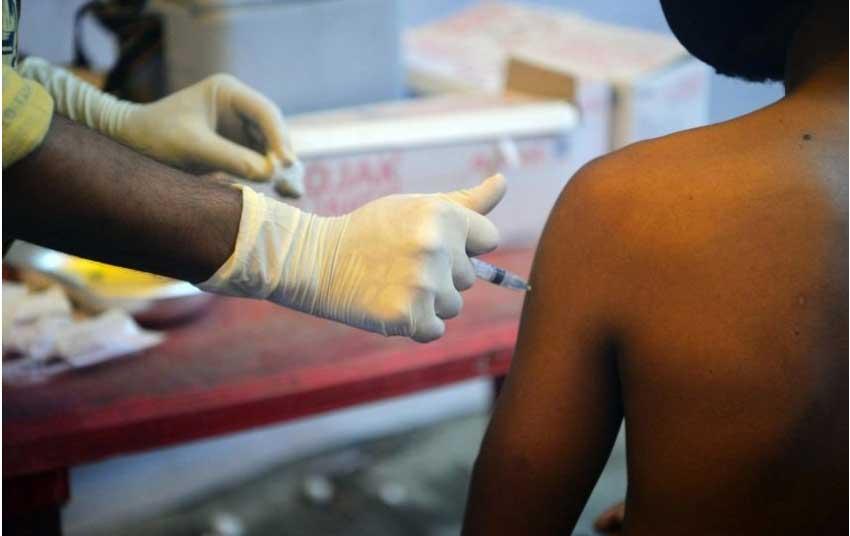
New Delhi, (Reuters), 3 June 2021 - India’s government signed its first purchase order for unapproved COVID-19 vaccines on Thursday, a day after it faced criticism from the top judiciary about a bungled vaccine rollout that has left millions of people vulnerable.
After a devastating second wave of infections that killed tens of thousands in April-May, the focus has shifted to urgently inoculating India’s vast adult population to curb infections later this year.
The government will buy 300 million doses from local firm Biological-E and has put down an advance of $205.6 million, the health ministry said, even though the vaccine is still undergoing phase-3 clinical trials, before approvals can be given.
India has been inoculating its people with the AstraZeneca vaccine produced locally at the Serum Institute of India (SII), Covaxin made by local firm Bharat Biotech and has begun rolling out Russia’s Sputnik V.
But supplies are running tight after the government opened vaccinations to all adults last month. Some vaccination centres have had to close down, prompting criticism from the Supreme Court about a lack of proper planning.
While the federal government gave free vaccines to the elderly and frontline workers, it left it to state governments and private hospitals to administer doses to people in the 18 to 45 age group at a pre-determined price.
It asked the government to review its vaccination policy, produce a roadmap and said the court could not be a silent spectator when the constitutional rights of the citizens were at risk.
15 Nov 2024 28 minute ago
15 Nov 2024 49 minute ago
14 Nov 2024 2 hours ago
14 Nov 2024 3 hours ago
14 Nov 2024 3 hours ago