29 Jan 2021 - {{hitsCtrl.values.hits}}
 The story of vaccines began when Edward Jenner inoculated a young boy to prevent smallpox using pus from cowpox blisters. This led to the production of an effective vaccine which contributed to the eradication of smallpox from the world nearly two centuries later.
The story of vaccines began when Edward Jenner inoculated a young boy to prevent smallpox using pus from cowpox blisters. This led to the production of an effective vaccine which contributed to the eradication of smallpox from the world nearly two centuries later.
The elimination of diseases since the days of ancient Egypt hasn’t been an easy task. Disease has been widespread and killed 300 million people in the 20th century alone. And in the 1950s there was an average of 50 million patients being recorded. However, in 1980 the World Health Organisation declared smallpox eradicated from this world. This was the first eradicated disease in the world and a monumental achievement of modern medicine. Achieving something of this magnitude could only be achieved by the union of different branches of science and logistics along with the cooperation of every single government in the world.
One such aspect that needs to be handled when dealing with such a widespread global disease is safety. As we all know, a vaccine is given to the people maintaining strict quality control and following extreme scientific methods. However, since billions of people need to be vaccinated there maybe rare instances of side effects which would still occur. Therefore, extreme vigilance and care are needed even after the vaccine is administered because safety issues can derail vaccine programmes worldwide. Experts in the field regularly monitor its quality, effectiveness, safety and seldom-occurring side effects. To monitor such safety issues a system called Adverse Events Following Immunization (AEFI) Surveillance has been implemented.
What is an AEFI?
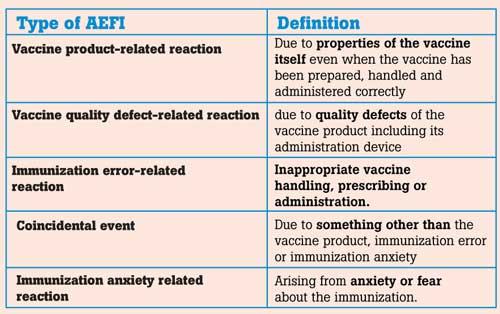
AEFI is “any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine.” According to this definition AEFI are categorised as follows
Even though all of these are called AEFI out of these 5 categories only the first two are vaccine reactions. They are also much rarer than the other categories. Immunisation error is preventable by proper training of the health staff. Coincidental events and anxiety related events make up most of the AEFI. However, since initially, we cannot determine which caused the AEFI at a glance; all aspects must be investigated and treated.
One such example is that of the 72-year-old volunteer who was struck by lightning after getting the Moderna’s COVID vaccine. Doctors investigated and diagnosed him with an irregular heart beat believed to have been caused by the strike.
Surveillance of AEFI
All serious and non-serious AEFI are reported to the Epidemiology Unit. The area MOH will investigate all reported serious cases of AEFI, while the Epidemiology Unit will investigate all deaths linked to immunization. All deaths suspected to be linked with immunization require a post-mortem investigation. Allergic reactions following immunization is also reported separately.
Based on those reports, investigations are carried out locally, nationally and internationally. Corrective actions are taken immediately to ensure the public safety.
This system applies to any new and old vaccine administered. Therefore, the health sector in this country is constantly on the lookout for your safety to ensure the trust you place in us and to maintain your health at the optimum level.
(The writer is a fourth year undergraduate at the Faculty of Medicine, University of Kelaniya)
21 Dec 2024 1 hours ago
21 Dec 2024 3 hours ago
21 Dec 2024 5 hours ago
21 Dec 2024 5 hours ago
21 Dec 2024 6 hours ago