16 Aug 2019 - {{hitsCtrl.values.hits}}
 industrialization increases stress in most of us every day in our lives. Sometimes our busy life schedules shoot our stress levels way too high. Under this stress, our body releases the cortisol hormone and too much cortisol can interfere with thyroid hormone production while it stimulates the thyroid to work harder to create sufficient amounts of thyroid hormone. Other than that, stress promotes vulnerability in us to autoimmune thyroid conditions such as, Hashimoto’s thyroiditis which is an autoimmune disease, in which the immune system attacks the thyroid. This can lead to hypothyroidism. Due to these stressful lifestyles and incorrect feeding patterns might be contributing to the new generation suffering from thyroid deficiency.
industrialization increases stress in most of us every day in our lives. Sometimes our busy life schedules shoot our stress levels way too high. Under this stress, our body releases the cortisol hormone and too much cortisol can interfere with thyroid hormone production while it stimulates the thyroid to work harder to create sufficient amounts of thyroid hormone. Other than that, stress promotes vulnerability in us to autoimmune thyroid conditions such as, Hashimoto’s thyroiditis which is an autoimmune disease, in which the immune system attacks the thyroid. This can lead to hypothyroidism. Due to these stressful lifestyles and incorrect feeding patterns might be contributing to the new generation suffering from thyroid deficiency.
Thyroid hormone
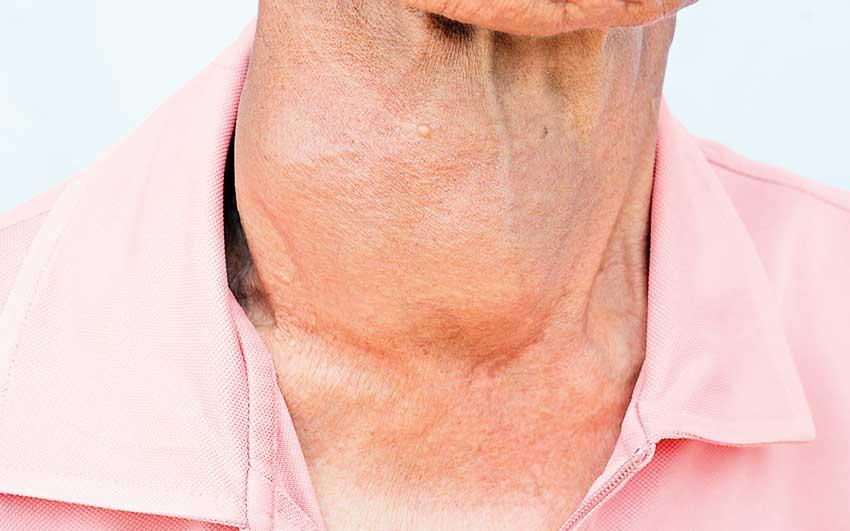
The thyroid hormone is produced in the thyroid gland which is a vital hormone gland with a butterfly-shape which is found at the front of the neck, under the voice box since it plays a major role in metabolism, growth and development of the human body. This is done by regulating many bodily functions via constantly releasing a steady amount of thyroid hormones into the bloodstream. The thyroid gland produces three hormones such as Triiodothyronine (T3), Tetraiodothyronine, (thyroxine or T4) and Calcitonin.
Function of thyroid hormones
Thyroid diseases
Thyroid disease is a disorder that affects the thyroid gland and as a result of that the body produces too much or too little thyroid hormone. Production of too much thyroid hormone by thyroid gland is called hyperthyroidism which causes to speed up many bodily functions. However the production of too little thyroid hormone by the gland is called hypothyroidism which causes many of the body’s functions to slow down.
Hypothyroidism
Hypothyroidism is thyroid hormone deficiency and there are two types namely Primary hypothyroidism and secondary hypothyroidism.
Primary Hypothyroidism
Primary hypothyroidism is occurred due to disease in the thyroid gland and the most common cause is autoimmune. It usually results from Hashimoto thyroiditis and is often associated with a firm goiter or, later in the disease process, with a shrunken fibrotic thyroid with little or no function. The second most common cause is post-therapeutic hypothyroidism, especially after radioactive iodine therapy or surgery for hyperthyroidism or goiter. As a result thyroid-stimulating hormone (TSH) is increased. Moreover, most of the patients with non-Hashimoto goiters have hyperthyroidism, but goitrous hypothyroidism may occur in endemic goiter due to iodine deficiency.
Secondary Hypothyroidism
Secondary hypothyroidism occurs when the hypothalamus produces insufficient thyrotropin-releasing hormone (TRH) or the pituitary produces insufficient TSH. Sometimes, deficient TSH secretion due to deficient TRH secretion is termed tertiary hypothyroidism.
Iron deficiency and Hypothyroidism
Iron metabolism is very intricately connected to thyroid hormone metabolism. Research has suggested that iron deficiency can contribute to the development of hypothyroidism. Normal thyroid status is dependent on the presence of many trace elements such as iron, iodine, selenium, and zinc for both the synthesis and metabolism of thyroid hormones and Iron is a component of many enzymes including thyroid peroxidase (TPO) which takes part in the initial two steps in thyroid hormone biosynthesis. The interrelationship between iron, red blood cells, and TSH can contribute to hypothyroidism by interfering with the normal function of the thyroid gland due to iron which is central to the production of both red blood cells and thyroid stimulating hormone (TSH). Estimation of serum ferritin (storage form), iron and total iron binding capacity (TIBC), which measures percent saturation of transport form transferrin with iron, may be of great significance in hypothyroidism.
Iodine and Hypothyroidism
Iodine is a trace element that is essential for the synthesis of thyroid hormones. However, both chronic iodine deficiency and chronically high iodine intake have been associated with the development of goiter and hypothyroidism attributed to excessive secretion of TSH by the pituitary. In turn, goiter has been associated with thyroid cancer risk, particularly in women. Iodine deficiency can cause inadequate thyroid hormone production in newborn infants which is called congenital hypothyroidism.
Causes for Hypothyroidism
Autoimmune thyroiditis (Hashimoto’s thyroiditis), Postpartum thyroiditis (a woman’s thyroid gland becomes inflamed after having a baby), anti-thyroid medications (e.g. amiodarone, lithium which inhibits hormones release by the thyroid), Iatrogenic causes (e.g. radioactive iodine, thyroidectomy), Congenital hypothyroidism (thyroid hormone deficiency present at birth caused by a problem with thyroid gland development or a disorder of thyroid hormone biosynthesis ), have a family history of thyroid disease or any autoimmune disease, Type 01 diabetes or rheumatoid arthritis, or other autoimmune disorders and experience of thyroid surgery (thyroid removed to treat thyroid cancer or to treat a symptomatic goiter)
Signs of thyroid deficiency
Some signs include Tiredness, weakness, fatigue, sleepiness, cold intolerance , roughness of voice, hair loss, constipation, joint pains and muscle cramps, depression, menorrhagia (menstrual periods with abnormally heavy or prolonged bleeding), infertility, weight gain, dry skin, puffy face, elevated blood cholesterol level, CPK (Creatine phosphokinase ), Hyponatremia ( low sodium concentration in the blood), Hyperprolactinemia (has higher-than-normal levels of prolactin hormone in the blood), slowed heart rate, impaired memory and enlarged thyroid gland (goiter)
Maternal Hypothyroidism
Pregnancy influences thyroid function in multiple ways. Not only does the maternal hypothalamic-pituitary-thyroid (HPT) axis undergo a series of adjustments, the fetus develops its own HPT axis and the placenta plays an active role in iodide and T4 transport and metabolism. Thus, an integrated three compartment thyroid model exists during pregnancy . In early stages of pregnancy, estrogen promotes production of a more highly T4-binding globulin isoform that is less rapidly degraded, resulting in increased serum T4-binding globulin and T4 concentrations. Although a transient decrease in serum free T4, followed by a rise in TSH to a new equilibrium, may occur. A high circulating HCG (human chronic gonadotropin) level in the first trimester leads to HCG cross-reactivity with the TSH receptor, prompting a temporary increase in free T4 and partial suppression of TSH. The final physiologic change results from placental de-iodination of maternal T4, which increases T4 turnover. In normal pregnant women, the thyroid gland maintains normal functioning of the thyroid with only minor fluctuations in serum T4 and TSH. However, in women with limited thyroid reserve, due to thyroid autoimmunity or iodine deficiency, hypothyroidism can develop.
Fetal thyroid ontogeny begins at 10–12 weeks gestation and is not complete until delivery; T4 is not secreted until 18–20 weeks. T4 is critical for many aspects of brain development including neurogenesis, neuronal migration, axon and dendrite formation, myelination, synaptogenesis, and neurotransmitter regulation. Although these requirements evolve over months, an especially critical time is the second trimester.
Thyroid hormones are crucial to fetal brain and nervous system development,. Uncontrolled hypothyroidism especially during the first trimester can affect the baby’s growth and brain development. In addition to that, uncontrolled hypothyroidism during pregnancy can lead to;
Preeclampsia (condition that develop in pregnant women and will develop high blood pressure and high protein in their urine.), anemia (prevents the body from getting enough oxygen since lack of red blood cells in blood), miscarriage, low birth weight of infant, still birth and congestive heart failure.
Hypothyroidism and Cardiovascular diseases
The most common cardiovascular signs and symptoms of hypothyroidism are abnormally slow heart action (bradycardia), mildhypertension (diastolic), narrowed pulse pressure, cold intolerance, and fatigue. Overt hypothyroidism decreased cardiac contractility, decreased cardiac output, and accelerated atherosclerosis and coronary artery disease. According to the research studies, hypothyroid patients have other atherosclerotic cardiovascular disease risk factors and an apparent increase in risk of stroke as well. The blood pressure changes, alterations in lipid metabolism, decreased cardiac contractility are caused by decreased thyroid hormone action on multiple organs such as the heart, liver, and peripheral vasculature and are potentially reversible with thyroid hormone replacement.
Cardiovascular Risks Associated With Hypothyroidism
Impaired cardiac contractility and diastolic function, increased systemic vascular resistance, decreased endothelial-derived relaxation factor, increased serum cholesterol, increased C-reactive protein, increased homocysteine (amino acid and high levels of it is linked to early development of heart disease).
Recommendation for Management of hypothyroidism
The writer is a medical laboratory technologist at a private hospital and holds a MSc. Degree in Industrial and Environmental Chemistry from the University of Kelaniya and a BSc. In Food Production and Technology and a Management degree from the Wayamba University of Sri Lanka.
21 Dec 2024 21 Dec 2024
21 Dec 2024 21 Dec 2024
21 Dec 2024 21 Dec 2024
21 Dec 2024 21 Dec 2024
21 Dec 2024 21 Dec 2024