20 Jan 2021 - {{hitsCtrl.values.hits}}
Moderna is currently testing its mRNA-1273 Coronavirus Efficacy vaccine on volunteers [AFP]
- The vaccine manufacturers started to produce these vaccines, while the trials were still going on, taking a huge risk of loss, if the trials failed
- Recently, many serious side effects of the vaccines were reported in the media, globally. This has caused anxiety and fear among many people about being vaccinated against COVID-19
- The genetic material of the SARS-CoV-2 is released and the muscle cells takes it up and makes the spike protein
According to the World Health Organization (WHO), 64 vaccines are currently in clinical developmental  stage while 173 vaccines are in the pre-clinical developmental stage. However, only the Pfizer/BioNtech vaccine has received emergency validation from the WHO. The other two vaccines currently being widely used is the strazeneca/Oxford vaccine and the Moderna vaccine.
stage while 173 vaccines are in the pre-clinical developmental stage. However, only the Pfizer/BioNtech vaccine has received emergency validation from the WHO. The other two vaccines currently being widely used is the strazeneca/Oxford vaccine and the Moderna vaccine.
The Differences in the three vaccines
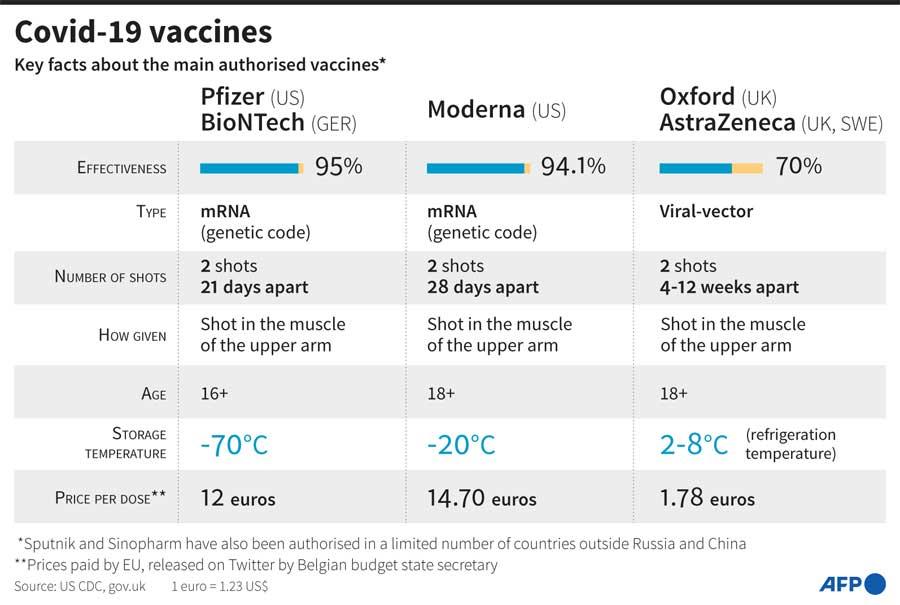
Speaking to Daily Mirror, Dr. Sanjaya Senanayake, Associate Professor at the Australian National University and an Infectious Diseases Specialist stated that the Moderna and Pfizer vaccines are the first mRNA vaccine, which is commercially available. “There has been research done on the possibility of mRNA vaccines for Influenza -- Zika viruses but it has never been commercially available,” he said. Dr. Senanayake explained that the spike protein of the virus is directly injected into the blood stream. “It is injected to the muscle cells in the arm. The muscle cells take up the genetic material and start making the spike protein. Then, the immune system encounters the spike protein and creates responses to it. This is how immunity is achieved,” he said. However, he pointed out that the mRNA vaccines are unstable and had to be transported under very cold temperatures. The Pfizer and Moderna vaccines have to be transported in -70°C and -20°C respectively.
“Once thawed out, the Pfizer vaccine can last in a fridge only for 5 day while the Moderna vaccine can last for about a month,” he said adding that for a poorly resourced country, it could be a challenge to transport and store these vaccines.
"The Pfizer vaccine’s two doses have to be taken 21 days apart while in both, the Moderna and Astrazeneca vaccines two doses have to be taken 28 days apart"
Explaining about the Astrazeneca/Oxford vaccine, Dr. Senanayake said the vaccine uses a technology that has already been in use before. “It uses a viral vector, which is a weakened chimpanzee adenovirus. This is loaded up with the spike protein of the SARS-CoV-2 virus and this is injected to the muscle cell. The genetic material of the SARS-CoV-2 is released and the muscle cells takes it up and makes the spike protein. When the immune system encounters the spike protein, it creates an immune response against it. Thus, immunity is achieved.” He said while researches tried to use the human adenovirus, the vaccine didn’t respond well. “This could be due to the human body recognizing the adenovirus and therefore killing it before it could carry out its mode of action,” Dr. Senanayake said and pointed out that the vaccine could be stored at 2°C- 8°C in the fridge, thus making it more temperature-friendly. He said the mRNA vaccines (Pfizer and Moderna) are 5 to 10 times more expensive than the Astrazeneca vaccine.
However, the Pfizer and Moderna vaccines are reported to have about 95% efficacy while the Astrazeneca vaccine has about 70% efficacy.
Dr. Senanayake said all the three vaccines: Pfizer, Moderna and Astrazeneca are double dose vaccines. The Pfizer vaccine’s two doses have to be taken 21 days apart while in both, the Moderna and Astrazeneca vaccines two doses have to be taken 28 days apart.
Concerns about the vaccines
“The shortest time taken for a vaccine to be developed before these COVID-19 vaccines were developed is the Mumps vaccine which was developed in four years, however due to COVID-19 being a global health emergency -- the vaccines were developed quickly,” he said adding that people need not be worried as proper clinical trials had been conducted.
Sharing similar sentiments, Prof. Neelika Malavige, Head of the Department of Immunology and Molecular Medicine at the University of Sri Jayawardenapura, said there was a huge global effort in developing the vaccines. “Therefore, all funding was directed towards their development. The evaluations of different phases of trials, which usually take months was done very fast as scientists around the world were working round the clock to get these out as soon as possible. The governments in these countries were putting all the money and effort into this. The vaccine manufacturers started to produce these vaccines, while the trials were still going on, taking a huge risk of loss, if the trials failed. Vaccine safety is the most important thing, and no short cuts were made. What you are seeing is what can be achieved when there is a huge commitment, adequate funding and a large team of capable individuals working to achieve a common goal,” she said.
Lifelong immunity?
Speaking about immunity, Dr Senanayake said there was still insufficient data to say how long the immunity will last. “For now, we can say it lasts for four months or so. But as it is administered, we will be able to figure out how long the immunity will last. If it lasts one year, we can say it lasted one year. If two years, we can say it lasted two years and so on,” he said. But Dr. Senanayake was hopeful that immunity may last a year or so, as recent researches have shown that the COVID-19 antibodies in people who were infected had lasted a few months.
Prof. Malavige said even people, who were infected with COVID-19 and had recovered should get vaccinated as there is no knowledge so far as to how long immunity will last, as a small proportion had been re-infected.
"No vaccine provides immunity to any virus, 7 to 10 days after receiving just one dose of a vaccine. This is the case for all vaccines currently given to humans and not just the COVID-19 vaccines"
Speaking about the recent news reports of people contracting COVID-19 even after being vaccinated, she said the double doses had to be completed to experience the full efficacy of the vaccine. “No vaccine provides immunity to any virus, 7 to 10 days after receiving just one dose of a vaccine. This is the case for all vaccines currently given to humans and not just the COVID-19 vaccines,” she said.
Prof. Malavige said most individuals started producing antibodies, 10 days after vaccination and it gradually increased with time. “The neutralising antibody responses (the important antibodies to prevent virus binding), peaked at 42 days after receiving the vaccine,” she said.
Common Side Effects
The common side effects in the Pfizer vaccine are headache and fatigue while fever and joint pain were seen in some of the patients. In the Moderna vaccine, site pain, fatigue, headache and muscle pain were common symptoms while few experienced nausea and facial swelling. In the Astrazeneca vaccine, the common side effects reported are site pain, slight fever, muscle ache and headache with some people experiencing arm pain and chills.
However, Dr. Senanayake noted that the Pfizer and Moderna vaccines have not been tested on pregnant women so there were no data as to how it would affect them.
Serious Side Effects
Recently, many serious side effects of the vaccines were reported in the media, globally. This has caused anxiety and fear among many people about being vaccinated against COVID-19. Speaking on this, Prof. Malavige said serious allergic reactions were reported after the administration of the Pfizer and Moderna vaccines. “The cause has not been identified with 100% certainty, but researchers believe the allergens could be the polyethylene glycol (PEG) or the lipid nanoparticles,” she said.
Prof. Malavige said the incidence of anaphylaxis is reported to be almost 10 times more than with other vaccines currently in use (data after immunising two million people in the USA). Anaphylaxis, which is a severe allergic reaction, was found to have occurred in those with a history of severe allergies. “The incidence of anaphylaxis is 1:100,000 instead of 1:1,000,000 reported for other vaccines. Therefore, those with severe allergic reactions are advised to get the vaccines only following medical advice,”
she advised.
The Differences in the three vaccines
 Dr. Sanjaya Senanayake, Associate Professor at the Australian National University and an Infectious Diseases Specialist - Dr Sanjaya Senanayake
Dr. Sanjaya Senanayake, Associate Professor at the Australian National University and an Infectious Diseases Specialist - Dr Sanjaya Senanayake
- There has been research done on the possibility of mRNA vaccines for Influenza -- Zika viruses but it has never been commercially available
- It is injected to the muscle cells in the arm. The muscle cells take up the genetic material and start making the spike protein. Then, the immune system encounters the spike protein and creates responses to it. This is how immunity is achieved
Serious Side Effects
 Head of the Department of Immunology and Molecular Medicine at the University of Sri Jayawardenapura - Prof Neelika Malavige
Head of the Department of Immunology and Molecular Medicine at the University of Sri Jayawardenapura - Prof Neelika Malavige
The cause has not been identified with 100% certainty, but researchers believe the allergens could be the polyethylene glycol (PEG) or the lipid nanoparticles
The incidence of anaphylaxis is 1:100,000 instead of 1:1,000,000 reported for other vaccines. Therefore, those with severe allergic reactions are advised to get the vaccines only following medical advice
27 Nov 2024 46 minute ago
27 Nov 2024 2 hours ago
27 Nov 2024 2 hours ago
27 Nov 2024 2 hours ago
27 Nov 2024 3 hours ago