09 Apr 2021 - {{hitsCtrl.values.hits}}
Experts observe lapses in COVID vaccine administration and documentation

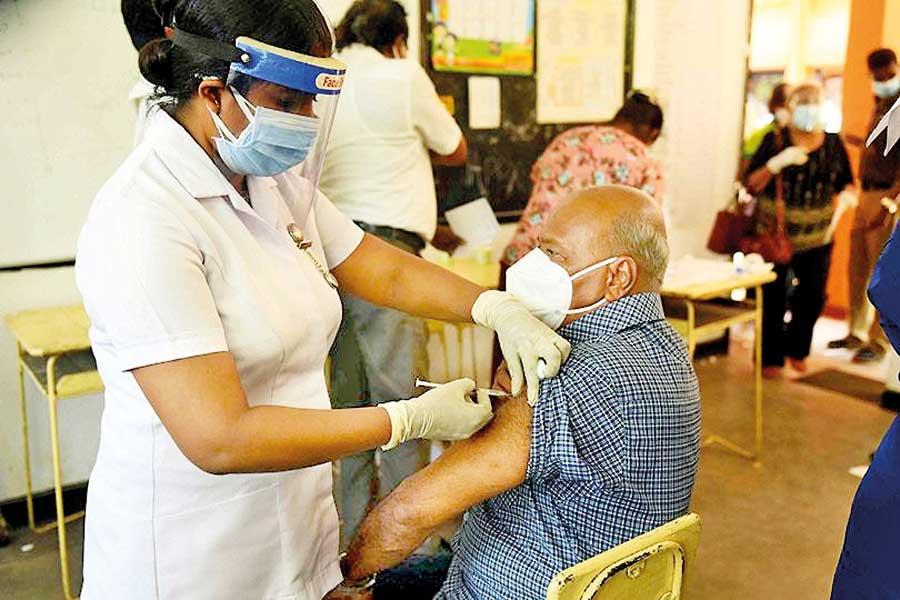
People gathered in large numbers to get vaccinated when Sri Lanka received the first set of vaccines
Sri Lanka commenced its COVID vaccination programme with much fanfare last January. Frontline healthcare workers, military personnel and those at immediate risk were vaccinated first. The government then decided to vaccinate people above 60. Subsequently, the government announced that it would vaccinate any and all people, even those below 30. Three months later, State Minister of Primary Healthcare, Epidemics and COVID Disease Control Sudarshani Fernandopulle announced that the COVID vaccination programme will be temporarily suspended as the government hadn’t been able to secure vaccines from Serum Institute of India. However, on the same day she reiterated that the vaccination drive would begin from April 19. Reportedly, a little over 900,000 people out of the 21 million people have so far been vaccinated. Apart from haphazard vaccination drives, medical experts also have raised concerns over haphazard approval processes that have been implemented in order to give clearance to certain vaccines.
healthcare workers, military personnel and those at immediate risk were vaccinated first. The government then decided to vaccinate people above 60. Subsequently, the government announced that it would vaccinate any and all people, even those below 30. Three months later, State Minister of Primary Healthcare, Epidemics and COVID Disease Control Sudarshani Fernandopulle announced that the COVID vaccination programme will be temporarily suspended as the government hadn’t been able to secure vaccines from Serum Institute of India. However, on the same day she reiterated that the vaccination drive would begin from April 19. Reportedly, a little over 900,000 people out of the 21 million people have so far been vaccinated. Apart from haphazard vaccination drives, medical experts also have raised concerns over haphazard approval processes that have been implemented in order to give clearance to certain vaccines.
Incomplete documentation creates a stir
When Sri Lanka received its first donation of Sinopharm vaccines, several medical experts raised concerns over incomplete documentation and certain technical issues. This prompted decision makers to sack few experts who opposed the approval process. However, the country’s drug regulator, the National Medicines Regulatory Authority (NMRA) is still in the process of getting the clearance to inoculate the public with the vaccine in question.
“When a country is accepting a donation from another country the receiving country should ensure that these items are firstly regulated in their country,” opined one of the experts who spoke on conditions of anonymity. “The country of origin should then present this information to the country that is receiving their products. If we are receiving some vaccines, then the NMRA will ask for a set of documents. These include Phase I-III trials, stability of the vial, safety and efficacy among other data.”
"When Sri Lanka received its first donation of Sinopharm vaccines, several medical experts raised concerns over incomplete documentation and certain technical issues"
“At least safety and efficacy data needs to be cleared. But the expert panel has observed that the submissions lack those data. All vaccines Sri Lanka received including Oxford AstraZeneca, Russian manufactured Sputnik were also approved adhering to this process. If the vaccine isn’t approved by the WHO or U.S Food and Drug Administration (FDA) all data related to Phase III clinical trials have to be submitted to the local regulator, NMRA.”
The panel of experts have raised concerns particularly because they haven’t received these data to date. “Granting approval without scrutinising such data would be a detrimental precedent. Every vaccine presented for approval should be approved only by adhering to the proper methodology.”
"A vaccine is administered to healthy, disease-free people. Therefore it is essential to approve a vaccine only after carefully evaluating its side effects and life-threatening effects"
The experts observe that a possible reason for the delay maybe that a large population is required to test the efficacy of a vaccine and by the time phase III trials began, China may have controlled the spread of the disease and may not have had sufficient number of patients. During a Phase III trial around 10,000-40,000 people or even more are vaccinated. They go through what is called a ‘double blind trial.’ Here you take 5000 people and vaccinate 2500 of them while giving a placebo to the remaining lot. Then you follow them for five or six months and wait until around 100 people get COVID. When they get a fever they are checked for COVID and this continues. When 100 people get COVID around 50 have gotten the vaccine. This means the vaccine isn’t effective. The level of antibodies among people who received the vaccine and those who didn’t also needs to be checked. This is what you call efficacy.
"Five critically important areas need to be considered when selecting a suitable vaccine. These include WHO approval, safety, efficacy, physical environment and logistics suitable for the vaccine and cost-effectiveness"
A vaccine is administered to healthy, disease-free people. Therefore it is essential to approve a vaccine only after carefully evaluating its side effects and life-threatening effects,” the expert added.
Vaccine mess
The Daily Mirror also learned that Sri Lankan government has paid for 1.5 million vaccine doses but has received only 500,000 vaccines. This means one million of them are pending. Around 240,000 doses have been given to the public. Another 264,000 vaccines were received under the COVAX facility. The WHO said that they were supposed to provide vaccines for 20% of the population which is around 8.4 million vaccines and later said that they would give vaccines to suffice 27% of the population. This is roughly 10 million vaccines. However Sri Lanka didn’t receive vaccines in March and is looking forward to receiving it this April. Meanwhile, Health Minister Pavithra Wanniarachchi said in Parliament yesterday that the second doses of Oxford AstraZeneca vaccines will be made available to the public from April 23 to July 6.
"Sri Lankan government has paid for 1.5 million vaccine doses but has received only 500,000vaccines. This means one million of them are pending."
Second dose could be given in three months
While stating that he was unaware of the circumstances that prompted the sacking of medical experts who opposed the approval of the Sinopharm vaccine, Committee on Public Accounts (COPA) Chairman Prof. Tissa Vitharana said that usually a committee of experts are appointed to decide on the vaccine that is best suited for the people. “They would decide on cost and other factors prior to distributing it to the public. There are important tests that need to be complete and experts need to be satisfied with the results. These are all technical aspects.”
When asked about the delay in getting the second dose of vaccines, Prof. Vitarana who is also a consultant virologist said that usually the booster is given 3-4 weeks after the first dose. “But studies conducted abroad have revealed that the booster level is optimal if the second dose is given within three months. A booster is given for maximum defense,” he added.
"Studies conducted abroad have revealed that the booster level is optimal if the second dose is given within three months. A booster is given for maximum defence"
Questions unanswered
“Those who got the first vaccine could wait up to 12 weeks as per available data,” opined Consultant Family Physician Dr. Ruvais Haniffa. “But we had our first dose in February. The two main concerns are with regard to clinical and logistical delays. The body reacts in certain ways and the second dose could be given within two to 12 weeks. Whether it could be given in three months is yet to be revealed as they haven’t updated data based on new studies as yet.”
"Right now we have only inoculated a fraction of people in Western Province but we need to vaccinate the entire population and it would roughly take around two years."
Observing the logistical delay, Dr. Haniffa said that the decision makers haven’t given any explanation as to why the second dose isn’t given as yet. “The first dose was given to frontline health workers and other individuals. So we do not know if we have run out of stocks or whether we couldn’t procure the vaccine or whether there is a genuine delay. Under the COVAX facility, the WHO was supposed to give us vaccines free-of-charge. But we received the first quota of vaccines and not the second. People still have first round immunity and this is also a matter that concerns the rights of people. Are we to demand for the second dose or are we at the mercy of the government?” he questioned.
"Those who got the first vaccine could wait up to 12 weeks as per available data
- Consultant Family Physician Dr. Ruvais Haniffa"
Emergency use approval
Expressing their concerns regarding the issuance of the Sinopharm vaccine, the Government Medical Officers’ Association (GMOA) in a statement said that five critically important areas need to be considered when selecting a suitable vaccine. These include WHO approval, safety, efficacy, physical environment and logistics suitable for the vaccine and cost-effectiveness. “Out of the above criteria, at least an Emergency Use Approval from WHO is required to ensure the vaccines’ safety and efficacy. The GMOA therefore requests relevant authorities to consider all of the above factors before vaccinating the public from a new vaccine brand.
"Sri Lanka received 1,264,000 vaccines from the covishield facility but India has suspended exporting vaccines due to the rapid spread of the virus."
- Ravi Kumudesh
Sri Lanka running out of vaccines?
“Sri Lanka received 1,264,000 vaccines from the COVISHIELD facility but India has suspended exporting vaccines due to the rapid spread of the virus,” opined Ravi Kumudesh, President of the Sri Lanka Association of Government Medical Laboratory Technologists. “It has always been said that when giving a first dose of a vaccine, the second dose also needs to be considered. Therefore if we received one million vaccines, only 500,000 should be given to the public. The entire world is running out of vaccines, even developed countries. There’s a speed at which vaccines are being developed. We have so far given around 900,000 vaccines out of the total and only around 200,000 are remaining. The second dose is particularly important during the formation of antibodies.”
He further said that when one vaccine is given it is not advisable to give another vaccine. “Therefore this is a deliberate mistake done by the government. What needs to be done is to get down the remaining doses of vaccines and continue the programme.”
Two years needed to vaccinate entire population
“We have completed giving more than half the number of vaccines we received,” said Chief Epidemiologist Dr. Sudath Samaraweera. “Those who got the first dose will get the second within 10-12 weeks. We will be able to give the second dose if we start getting them from next month. Right now we have only inoculated a fraction of people in Western Province but we need to vaccinate the entire population and it would roughly take around two years.”
"We have completed giving more than half the number of vaccines we received
- Chief Epidemiologist Dr. Sudath Samaraweera"
When asked about vaccines the country is supposed to receive under the COVAX facility, Dr. Samaraweera said that they haven’t received a confirmation as to when they would receive it.
He further said that the Sinopharm vaccine is still being given to Chinese nationals and once a stringent regulatory authority assess information on the safety of the vaccine and issues a clearance, that they will start giving it to the public.
Several attempts to contact State Minister of Production, Supply and Regulation of Pharmaceuticals Prof. Channa Jayasumana proved futile.
27 Nov 2024 11 minute ago
27 Nov 2024 18 minute ago
27 Nov 2024 59 minute ago
27 Nov 2024 3 hours ago
26 Nov 2024 26 Nov 2024