04 Aug 2017 - {{hitsCtrl.values.hits}}

It has become nothing but humdrum when it comes to warning the general public in taking precautions against dengue. Something that we all know, despite every measure being taken, is that the disease can’t be eradicated overnight. This has to be said in the backdrop of the inevitable number of citizens falling victim to dengue everyday. So the situation has now aggravated and more attention is being paid to dengue cures and treatments. What has become really important is to find light at the end of the tunnel and that amounts to a medically curative method.
 The good news is that the world’s first licensed dengue vaccine, approved by the World Health Organization, is available and now in use as a treatment for dengue. The vaccine is currently been used in 18 countries. It’s also available in Asian countries such as Singapore, Malaysia, Indonesia and Thailand apart from being used in other continents. The licensed dengue vaccine is a live attenuated recombinant tetravalent vaccine which is given on a 3 dose series on a 0, 6 and 12 month basis. The vaccine is recommended for people in the age range of 9 - 45 years in dengue endemic countries.
The good news is that the world’s first licensed dengue vaccine, approved by the World Health Organization, is available and now in use as a treatment for dengue. The vaccine is currently been used in 18 countries. It’s also available in Asian countries such as Singapore, Malaysia, Indonesia and Thailand apart from being used in other continents. The licensed dengue vaccine is a live attenuated recombinant tetravalent vaccine which is given on a 3 dose series on a 0, 6 and 12 month basis. The vaccine is recommended for people in the age range of 9 - 45 years in dengue endemic countries.
Perspective of Medical Personas
According to Professor in Medicine Arjuna De Silva, attached to the Faculty of Medicine in Ragama, the dengue vaccine seemingly is an effective method to help control dengue in the country. “The vaccine is WHO approved. The vaccine has an overall effect against all four dengue serotypes. This seems to have highly probable positive effects to help treat dengue patients and so far has been reported safe. Also 7 of the Asian countries have implemented the vaccine,” Professor De Silva said.
While a vaccine for dengue seems so essential for a country like Sri Lanka-which is now battling a dengue crisis-declaring the use of this vaccine has not been simple, hence the delay in it being used in medical practice in the country. For the record around 300 dengue related deaths have been reported.
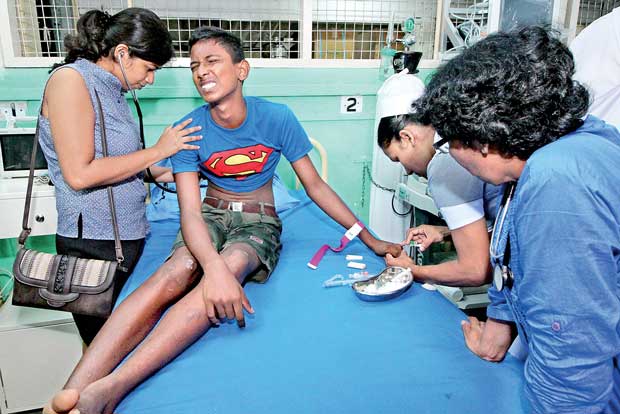
A dengue patient receives treatment at a government hospital
When the reasons for the delay in the dengue vaccine were inquired from the GMOA Secretary, Dr. Haritha Aluthge, he stated that the vaccine is yet to be studied and trial tested. Aluthge said that it is only then that it can be approved for medical use in the country. He said that such trials and tests are essentianl as a possible risk entails the implementation of a new vaccine. “Before the vaccine can be used on patients, a trial test has to be done to ensure safety. We should also refer to the trial tests done in other countries. The vaccine has to be studied and trial tested before it can be used medically. There is a procedure to follow with regard to the National Medical Regulatory Authority (NMRA) of the country before approving a vaccine. The vaccines should be affirmed in terms of cost effectiveness as well. We should also consider the success rate of the vaccine in other countries. Generally the procedure to introduce a new vaccine to a country is something that takes time. We can’t use Sri Lankan patients as guinea pigs,” he warned.
Reports reveal that Sri Lankans have flown to Singapore on various occasions to get the vaccine. While opulent citizens may be able to get vaccinated, the less affluent have been left without such an opportunity in obtaining the vaccination. These happenings have led to the questioning about the delay in the use of the vaccine in Sri Lanka given that it’s already being used in a country like Singapore.
But according to Dr. Aluthge, the results of the vaccine can change from country to country. That is why trial tests are necessarily. “The dengue vaccine is a new vaccine which hasn’t been used on patients for a long time. The vaccine doesn’t have a history of being used in medical practice for more than 20 or 30 years. So it’s a risk. There are instances where medicine that works for one country hasn’t proven successful in another. So a trial test has to be done on a scientific basis before officially introducing the vaccine to a country. It has to be decided whether the trial is going to be performed islandwide or in a selected province. There is an advisory committee with regard to national vaccines. The decision to be made with regard to this vaccine is up to this committee. Rather than talking about this topic while being in the dark, it’s better to obtain approval for the vaccine through a methodical manner,” he said.
“On behalf of the GMOA, what I can say is, if the vaccine is to be implemented, it has to be done using a scientific basis with respect to the laws of the country and it has to be done without unnecessary delay. The vaccine has to be passed as a national policy following the act of the NMRA with its approval,” he further said.
Straight from the Source
The approached Sanofi Pasteur, to obtain details of the vaccine. Sanofi Pasteur is the vaccines division of the French multinational pharmaceutical company, Sanofi , deemed as one of 4 companies that produces the vaccine for yellow fever globally.
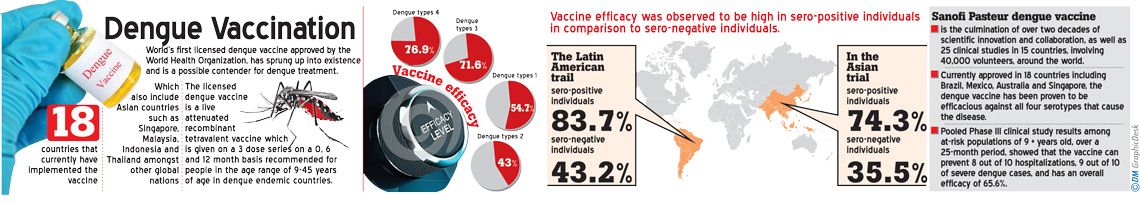
Spokesperson for Sanofi Pasteur referred to background details of the vaccine which include the countries that have used the vaccine. The overall effectiveness of the vaccine in treating the dengue disease was specified. “Sanofi Pasteur dengue vaccine is the culmination of over two decades of scientific innovation and collaboration, as well as 25 clinical studies in 15 countries, involving 40,000 volunteers, around the world. Currently approved in 18 countries including Brazil, Mexico, Australia and Singapore, the dengue vaccine has been proven to be efficacious against all four serotypes that cause the disease. Pooled Phase III clinical study results among at-risk populations of 9 + years old, over a 25-month period, showed that the vaccine can prevent 8 out of 10 hospitalizations, 9 out of 10 of severe dengue cases and has an overall efficacy of 65.6%,” the spokesperson said.
 The vaccine has an overall effect against all four dengue serotypes. This vaccine so far has been reported safe - Professor, Arjuna De Silva
The vaccine has an overall effect against all four dengue serotypes. This vaccine so far has been reported safe - Professor, Arjuna De Silva
When the company spokesperson was asked about their approach with the vaccine to Sri Lanka and the Sri Lankan response to the vaccine proposal, the spokesperson stated that the company had submitted an application to the NMRA for approval in June last year. The NMRA in response had informed the necessity of the assessment of surveillance data during a time period of one year from countries where the vaccine was implemented before it (vaccine) can be approved. “We submitted an application to obtain marketing approval to the Sri Lankan regulatory authorities in June 2016. In March 2017, the NMRA informed us that they would like to assess post-marketing surveillance data from countries in which the vaccine has been used for a period of 1 year before making a decision on approval of the vaccine. Sanofi Pasteur responded to the queries of NMRA in a letter dated April 4th, 2017 and provided long-term safety and efficacy data from clinical trials involving over 35,000 subjects and post-marketing safety surveillance. Since then, we have been closely engaged with the NMRA and await their decision on approval of the vaccine for use in Sri Lanka,” the spokesperson further said.
 Generally the procedure to introduce a new vaccine is something that takes time. We can’t use Sri Lankan patients as guinea pigs - GMOA Secretary, Dr. Haritha Aluthge
Generally the procedure to introduce a new vaccine is something that takes time. We can’t use Sri Lankan patients as guinea pigs - GMOA Secretary, Dr. Haritha Aluthge
The spokesperson stressed on the vaccine’s performance and effectiveness accentuating its safety and high efficacy. “We have long-term data on the safety of our vaccine for 6 years post vaccination from a Phase IIB efficacy study involving 4002 subjects and for 5 and 4 years respectively from two phase III studies conducted in 10 dengue-endemic countries inAsia and Latin America involving more than 31,000 subjects. The results consistently showed a continued reduction of hospitalized dengue cases. In addition, 2 Periodic Benefit-Risk Evaluation Reports have been published in between a 6-months interval since the post-marketing approval. These include safety data collected by Sanofi Pasteur from worldwide sources, since the vaccine’s approval in December 2015 till 07 December 2016, and do not warrant any significant Regulatory Authority actions.(e.g., restrictions in approved indications, suspensions or withdrawal of a marketing authorization, new contraindications for use, new or strengthened warnings),” the spokesperson said.
 The company believes that the vaccine will help make a difference in the dengue situation in the country, all the while assuring its safety and efficacy. “The long-term data on our vaccine and the post-marketing safety surveillance reassure us of the long-term efficacy and safety of the vaccine.
The company believes that the vaccine will help make a difference in the dengue situation in the country, all the while assuring its safety and efficacy. “The long-term data on our vaccine and the post-marketing safety surveillance reassure us of the long-term efficacy and safety of the vaccine.
This new tool will have a significant impact on our fight against dengue, when used along with other dengue control measures.Sanofi Pasteur stands ready to provide the vaccine to Sri Lanka and believes that this vaccine, coupled with other preventive measures, can make a difference in Sri Lanka in fighting this terrible disease,” the spokesperson added.
A much provoked inquiry
The question of the delay with regard to the implementation of the vaccine in Sri Lanka is a much provoked inquiry in many a mind. But in actuality this query must be rephrased to questioning the suitability of the vaccine to be used on Sri Lankans. The question, ‘Are we ready for the dengue vaccine to be used in Sri Lanka?’ was addressed in the Weekly Epidemiological Report issued by the Epidemiology Unit for March 18- 24 2017 It accentuated the necessity to approach the implementation of a vaccine program using a scientific approach. The report stressed that the current disease surveillance system in Sri Lanka is inadequate to assess dengue transmission dynamics at a national and sub national level. “The current surveillance system doesn’t capture morbidity data from out-patient departments, laboratories and the community. Therefore the patients who don’t get admitted to hospitals, particularly those with mild symptoms or clinically unapparent infections are unlikely to be reported and aren’t reflected in the morbidity figures. From the inpatients too, a proportion of cases may be missed from hospitals in different parts of the country. Consequently, under reporting is a significant problem with the routine surveillance mechanism currently in place,” the report reveals.
 The importance of an assessment to evaluate the vulnerable ages and the serological extent of dengue transmission has also been highlighted in the epidemiology report, in attempts to recognize the state of the dengue burden. “It is important that we have an understanding of the sero-prevalence of dengue in the country or at least in the high- risk areas like western province or in the Colombo district. In this back drop, a community based descriptive study of dengue sero-epidemiology in the Colombo district is being carried out by the epidemiology unit. Age-specific dengue sero-prevalence in the metropolitan, urban and rural populations in the Colombo district will be assessed in this study,” the report states. The study will prove helpful as age-stratified sero-surveys are currently deemed as the best way to select populations suitable for vaccination. When this is directed at a sub national level, it will help guide vaccine decision making. The Epidemiology Report stated that this knowledge is acclaimed important for evidence based effective control and as preventive strategies for future introduction of the vaccine. The outcome of this study is said to be important in accurately sizing up the dengue dilemma and preventing it from wreaking havoc in the country.
The importance of an assessment to evaluate the vulnerable ages and the serological extent of dengue transmission has also been highlighted in the epidemiology report, in attempts to recognize the state of the dengue burden. “It is important that we have an understanding of the sero-prevalence of dengue in the country or at least in the high- risk areas like western province or in the Colombo district. In this back drop, a community based descriptive study of dengue sero-epidemiology in the Colombo district is being carried out by the epidemiology unit. Age-specific dengue sero-prevalence in the metropolitan, urban and rural populations in the Colombo district will be assessed in this study,” the report states. The study will prove helpful as age-stratified sero-surveys are currently deemed as the best way to select populations suitable for vaccination. When this is directed at a sub national level, it will help guide vaccine decision making. The Epidemiology Report stated that this knowledge is acclaimed important for evidence based effective control and as preventive strategies for future introduction of the vaccine. The outcome of this study is said to be important in accurately sizing up the dengue dilemma and preventing it from wreaking havoc in the country.
Vaccine Technicality
 Referring to the Weekly Epidemiology Report (Vol.44, No.12) issued by the Epidemiology Unit for the time period between March 18-24, 2017, one dengue vaccine has been registered in several countries while there are several other vaccines for dengue being developed at the moment.
Referring to the Weekly Epidemiology Report (Vol.44, No.12) issued by the Epidemiology Unit for the time period between March 18-24, 2017, one dengue vaccine has been registered in several countries while there are several other vaccines for dengue being developed at the moment.
When it came to effectiveness of the vaccine in the trials, it has varied from each individual and also the country. “Vaccine efficacy varied from country to country in the studies, ranging from 31.3% in Mexico to 79% in Malaysia,” the report states. It was also further explained in the report that age and various scientific reasons such as sero-prevalence affected the effectiveness of the vaccine. Age and sero-positivity have depicted a higher correlation in the trials.
It was further reported that during vaccine studies, an instance of an increased risk of hospitalization for dengue was seen particularly in the age group of 2-5 year olds. There have been several hypotheses to suggest the cause with regards to medical science, but nothing has been confirmed. This result led to the decision of vaccinating patients starting from the age of 9 years. The sero-positivity or sero-negativity of a dengue patient seems to have an effect on an individual’s response to the dengue vaccine. “Vaccination may be ineffective or may theoretically even increase the future risk of dengue illness in those who are sero-negative at the time of first vaccination regardless of age. If this is the case, even in high transmission settings there may be an increased risk among sero-negative persons despite a reduction in dengue illness at the population level,” the report further states. A different report revealed that vaccine efficacy was high against dengue types 3(71.6%) and 4 (76.9%) in comparison to dengue types 1 (54.7%) and 2 (43%). Vaccine efficacy was observed to be high in sero-positive individuals in comparison to sero-negative individuals. In the Asian trial of the vaccine, the efficacy for sero-positive individuals was 74.3% whereas the efficacy was 35.5% for sero-negative individuals. The Latin American trail begged to differ with higher efficacies with sero-positive acquiring 83.7% and sero-negative efficacy being 43.2%.
The current surveillance system doesn’t capture morbidity data from out-patient departments, laboratories and the community
The Weekly Epidemiological Report also stated that mathematical models have been developed under various assumptions to predict the impact of dengue vaccines when administered in a routine immunization programme. The cost-effectiveness of the vaccines were also considered at in the modelling comparison. “As the cost of vaccine procurement and delivery was unknown, the analyses were presented as costs per fully vaccinated person. One DALY averted was valued at around US$ 2000 based on benchmarking the costs against alternative interventional strategies being carried out to prevent dengue. Against this benchmark, in settings with sero-prevalence in the range of 50%–90% at age 9 years, vaccination was predicted to be cost effective if the total cost of fully vaccinating one person were less than US$15–40 in the public health perspective. It should be noted, however, that the modelling comparison results were based on regional indicators and should be used as a substitute for country-specific analyses to effect local decision-making.”
 WHO has specified on the target groups for the vaccines. “In defining populations to be targeted for vaccination, prior infection with dengue virus of serotype, as measured by sero-prevalence, should be approximately 70% greater, in the age group targeted for vaccination, in order to maximize public health impact and cost effectiveness.” A different report stated that vaccination of populations in between the recommended age groups with sero-prevalence in the range between 50-70% is acceptable, but the impact may be lower than the desired level while the vaccination of populations with sero-prevalence below 50% is not recommended.
WHO has specified on the target groups for the vaccines. “In defining populations to be targeted for vaccination, prior infection with dengue virus of serotype, as measured by sero-prevalence, should be approximately 70% greater, in the age group targeted for vaccination, in order to maximize public health impact and cost effectiveness.” A different report stated that vaccination of populations in between the recommended age groups with sero-prevalence in the range between 50-70% is acceptable, but the impact may be lower than the desired level while the vaccination of populations with sero-prevalence below 50% is not recommended.
The Weekly Epidemiological Report further stressed that positive effects can’t be expected from the vaccine alone and that it should be considered part of a dengue control mechanism. “Dengue vaccination introduction should be carried out as a part of comprehensive dengue control strategy, including well-executed and sustained vector control, evidence based best practices for clinical care for all patients with dengue illness, and strong dengue surveillance,” the report stresses.
However it was also noted that though the dengue vaccination may be introduced as part of an overall dengue control strategy, it’s not expected to make a significant impact in the wake of an outbreak.
 The debate regarding the vaccine was taken to the streets when the Dailymirror made inquiries from the public to note down their opinions. This was mainly because we think that ultimately it’s the general public that must be the beneficiaries of a medical scheme that’s put to use. Citizens expressed a rainbow of views that included a dislike for the vaccine as well as those who supported it. Some opined that the implementation of a vaccine for dengue would be a good step while others harboured fears regarding the safety of the vaccine.
The debate regarding the vaccine was taken to the streets when the Dailymirror made inquiries from the public to note down their opinions. This was mainly because we think that ultimately it’s the general public that must be the beneficiaries of a medical scheme that’s put to use. Citizens expressed a rainbow of views that included a dislike for the vaccine as well as those who supported it. Some opined that the implementation of a vaccine for dengue would be a good step while others harboured fears regarding the safety of the vaccine.
“I completely agree with the implementation of a dengue vaccine. But I also think that the vaccine should be reinforced along with medical recommendations of a doctor,” said Wathsala.
 “I am all for the implementation of a dengue vaccine under safety assurances. I think it will prove beneficial as a treatment for dengue which is a dreadful disease,” said Manosha Fernando
“I am all for the implementation of a dengue vaccine under safety assurances. I think it will prove beneficial as a treatment for dengue which is a dreadful disease,” said Manosha Fernando
 “No, I don’t agree with a vaccine. Implementation of a vaccine isn’t child’s play. It could lead to dangerous consequences. Rather than approaching a vaccine, I think that there are many other actions that must be taken to suppress the dengue condition in the country. Though these vaccines are said to be used in countries like Singapore, we can’t ignore the fact that they are developed countries unlike Sri Lanka. So the success of the vaccine here is questionable. But if the vaccine is approved by doctors, then it could be a considerable step,” said Christaline Fernando
“No, I don’t agree with a vaccine. Implementation of a vaccine isn’t child’s play. It could lead to dangerous consequences. Rather than approaching a vaccine, I think that there are many other actions that must be taken to suppress the dengue condition in the country. Though these vaccines are said to be used in countries like Singapore, we can’t ignore the fact that they are developed countries unlike Sri Lanka. So the success of the vaccine here is questionable. But if the vaccine is approved by doctors, then it could be a considerable step,” said Christaline Fernando
 “The fact that the vaccine is being used in other countries is assuring. After all, most of the medicines used in Sri Lanka are from other countries as well. In any case, the vaccine would be a good thing. It would be good for the children,” said Ishan Perera
“The fact that the vaccine is being used in other countries is assuring. After all, most of the medicines used in Sri Lanka are from other countries as well. In any case, the vaccine would be a good thing. It would be good for the children,” said Ishan Perera
 “As a mother of two daughters, I am concerned for their safety, so I don’t agree with a vaccine if it isn’t approved by the Health Ministry and medical professionals. My daughters just recovered from dengue and I know the severity of the disease, but even so I won’t opt for a vaccine unless it’s approved medically in Sri Lanka,” said Niranjala
“As a mother of two daughters, I am concerned for their safety, so I don’t agree with a vaccine if it isn’t approved by the Health Ministry and medical professionals. My daughters just recovered from dengue and I know the severity of the disease, but even so I won’t opt for a vaccine unless it’s approved medically in Sri Lanka,” said Niranjala
23 Dec 2024 9 minute ago
23 Dec 2024 2 hours ago
23 Dec 2024 2 hours ago
23 Dec 2024 3 hours ago
23 Dec 2024 3 hours ago